��Ŀ����
A��B��C��D��E���ֶ�����Ԫ�ص�ԭ��������������������֮��Ϊ40��A��Dͬ���壬B��Cͬ���ڣ�A��B��ɵĻ�����Ϊ���壬����������ˮ�Լ��ԣ�A��C���γ�����Һ̬������A2C��A2C2��E�ǵؿ��к������Ľ���Ԫ�أ��Իش�
��1��д������Ԫ�ط��ţ�A ��B ��D ��
��2��������A2C�ĵ���ʽ�� �����õ���ʽ��ʾ������D2C���γɹ��� ��
��3��E������Fe��ϡ���ṹ�ɵ�ԭ����У�Fe�� ��������һ���缫�ϵĵ缫��ӦΪ ��
��4����D����Ͷ��A2C�е����ӷ���ʽΪ ��
��1��д������Ԫ�ط��ţ�A
��2��������A2C�ĵ���ʽ��
��3��E������Fe��ϡ���ṹ�ɵ�ԭ����У�Fe��
��4����D����Ͷ��A2C�е����ӷ���ʽΪ
���㣺λ�ýṹ���ʵ����ϵӦ��
ר�⣺Ԫ����������Ԫ�����ڱ�ר��
������A��B��C��D��E���ֶ�����Ԫ�ص�ԭ��������������������֮��Ϊ40��A��Dͬ���壬B��Cͬ���ڣ�A��B��ɵĻ�����Ϊ���壬����������ˮ�Լ��ԣ���û�������NH3��A��ԭ������С��B����A��HԪ�ء�B��NԪ�أ�A��Dͬһ���壬��D��ԭ����������B������D��NaԪ�أ�
A��C���γ�����Һ̬������A2C��A2C2��C��ԭ����������B��С��D����C��OԪ�أ�
E�ǵؿ��к������Ľ���Ԫ�أ���E��AlԪ�أ�
�ٽ�����ʽṹ�����ʽ��
A��C���γ�����Һ̬������A2C��A2C2��C��ԭ����������B��С��D����C��OԪ�أ�
E�ǵؿ��к������Ľ���Ԫ�أ���E��AlԪ�أ�
�ٽ�����ʽṹ�����ʽ��
���
�⣺A��B��C��D��E���ֶ�����Ԫ�ص�ԭ��������������������֮��Ϊ40��A��Dͬ���壬B��Cͬ���ڣ�A��B��ɵĻ�����Ϊ���壬����������ˮ�Լ��ԣ���û�������NH3��A��ԭ������С��B����A��HԪ�ء�B��NԪ�أ�
A��Dͬһ���壬��D��ԭ����������B������D��NaԪ�أ�
A��C���γ�����Һ̬������A2C��A2C2��C��ԭ����������B��С��D����C��OԪ�أ�
E�ǵؿ��к������Ľ���Ԫ�أ���E��AlԪ�أ�
��1��ͨ�����Ϸ���֪��A��HԪ�ء�B��NԪ�ء�D��NaԪ�أ��ʴ�Ϊ��H��N��Na��
��2��������H2O�ĵ���ʽ�� ��D2C��Na2O������ʽ��ʾ������Na2O���γɹ���
��D2C��Na2O������ʽ��ʾ������Na2O���γɹ���  ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
�� ��
��
��3��Al������Fe��ϡ���ṹ�ɵ�ԭ����У�Al��ʧ������������Fe�������������ϵ缫��ӦʽΪ���ʴ�Ϊ��Al-3e-=Al3+���ʴ�Ϊ������Al-3e-=Al3+��
��4��D��Na��A2C��ˮ�����߷�Ӧ�����������ƺ��������������ӷ���ʽΪ2Na+2H2O�T2Na++2OH-+H2����
�ʴ�Ϊ��2Na+2H2O�T2Na++2OH-+H2����
A��Dͬһ���壬��D��ԭ����������B������D��NaԪ�أ�
A��C���γ�����Һ̬������A2C��A2C2��C��ԭ����������B��С��D����C��OԪ�أ�
E�ǵؿ��к������Ľ���Ԫ�أ���E��AlԪ�أ�
��1��ͨ�����Ϸ���֪��A��HԪ�ء�B��NԪ�ء�D��NaԪ�أ��ʴ�Ϊ��H��N��Na��
��2��������H2O�ĵ���ʽ��
 ��D2C��Na2O������ʽ��ʾ������Na2O���γɹ���
��D2C��Na2O������ʽ��ʾ������Na2O���γɹ���  ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
�� ��
����3��Al������Fe��ϡ���ṹ�ɵ�ԭ����У�Al��ʧ������������Fe�������������ϵ缫��ӦʽΪ���ʴ�Ϊ��Al-3e-=Al3+���ʴ�Ϊ������Al-3e-=Al3+��
��4��D��Na��A2C��ˮ�����߷�Ӧ�����������ƺ��������������ӷ���ʽΪ2Na+2H2O�T2Na++2OH-+H2����
�ʴ�Ϊ��2Na+2H2O�T2Na++2OH-+H2����
���������⿼����Ԫ��λ�ýṹ���ʵĹ�ϵ��Ӧ�ã��漰���ӷ���ʽ����д��ԭ���ԭ��������ʽ����д��֪ʶ�㣬����Ԫ�����ڱ���ԭ�ӽṹȷ��Ԫ�أ���ȷ�ƶ�Ԫ���ǽⱾ��ؼ�����Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
���й���ԭ��ص���������ȷ���ǣ�������
| A����þ������ϡNaOH��Һ��ɵ�ԭ����У�þ�Ǹ������������� |
| B������������ϡ������ɵ�ԭ����У���������Ƭͨ������������Ƭ |
| C����п��ͭ��ϡ������ɵ�ԭ����У�����ع���ʱ������������������ƶ� |
| D����п��ͭ��CuSO4��Һ��ɵ�ԭ����У������������ᣬ������������ |
�������ʷ�Ӧʱ���������H2 ���ǣ�������
| A�����ȵ�����ˮ���� |
| B��Al��ϡ���� |
| C��Fe��ϡHNO3 |
| D��Al ��NaOH��Һ |
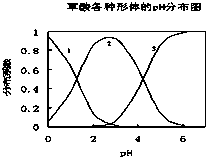 ���ᣨH2C2O4����һ��������ˮ�Ķ�Ԫ��ǿ�ᣬ��ˮ�����Ĵ�����̬��H2C2O4��HC2O4-��C2O42-������̬�ķֲ�ϵ����Ũ�ȷ�����������ҺpH�仯�Ĺ�ϵ��ͼ��ʾ��
���ᣨH2C2O4����һ��������ˮ�Ķ�Ԫ��ǿ�ᣬ��ˮ�����Ĵ�����̬��H2C2O4��HC2O4-��C2O42-������̬�ķֲ�ϵ����Ũ�ȷ�����������ҺpH�仯�Ĺ�ϵ��ͼ��ʾ�� ʵ�����ù���NaOH����0.5mol/L��NaOH��Һ500mL����������������Ʒ�У�
ʵ�����ù���NaOH����0.5mol/L��NaOH��Һ500mL����������������Ʒ�У�