��Ŀ����
�̷���FeSO4?7H2O�����ᷨ����һ��ϡ�н�����Ʒ�����в����ĸ���Ʒ����Ʒ���Ϊ����ɫ����ɫ�ᾧ���壮���������ɵ��ڼ���ˮ�е�pH����ˮ���������л���ϣ������ٳ�������ҪӦ����ˮ�ʾ�����ҵ��ˮ������ͬʱ����ɱ�����ã�
��1��98% 1.84g/cm3��Ũ������ϡ�����У��ܶ��½�����ϡ����50%ʱ���ܶ�Ϊ1.4g/cm3��50%���������ʵ���Ũ��Ϊ ��������λС������50%��������30%������������ϣ�������Ũ��Ϊ ���������=��40%��
��2��ʵ��������20%�������ᣨ100�˷������ẬSO320�ˣ�����ϡ���ᣬ����SO3?nH2O��ʾ20%�ķ������ᣬ��n= ��������λС������
��3���̷��ڿ��������ױ���������Ϊ����������ȡ7.32�˾�������ϡ�������������BaCl2��Һ�����˵ó���9.32�ˣ���ͨ��112mL����״��������ǡ�ý�Fe2+��ȫ�������Ʋ⾧��Ļ�ѧʽΪ ��
��4�����������[��NH4��2SO4?FeSO4?6H2O]���׳�Ī���Σ������̷��ȶ����ڷ�����ѧ�г���������Fe2+�ı���Һ���ô�Fe2+�ı���Һ���Բⶨʣ��ϡ�����������ȡ8.64��Cu2S��CuS�Ļ������200mL 2mol/Lϡ������Һ������������Ӧ���£�
10NO3-+3Cu2S+16H+=6Cu2++10NO��+3SO42-+8H2O
8NO3-+3CuS+8H+=3Cu2++3SO42-+8NO��+4H2O
ʣ���ϡ����ǡ����V mL 2mol/L ��NH4��2Fe��SO4��2��Һ��ȫ��Ӧ��
��֪��NO3-+3Fe2++4H+=NO��+3Fe3++2H2O
��Vֵ��Χ ��
����V=48���Լ���������CuS���������� ��������λС������
��1��98% 1.84g/cm3��Ũ������ϡ�����У��ܶ��½�����ϡ����50%ʱ���ܶ�Ϊ1.4g/cm3��50%���������ʵ���Ũ��Ϊ
��2��ʵ��������20%�������ᣨ100�˷������ẬSO320�ˣ�����ϡ���ᣬ����SO3?nH2O��ʾ20%�ķ������ᣬ��n=
��3���̷��ڿ��������ױ���������Ϊ����������ȡ7.32�˾�������ϡ�������������BaCl2��Һ�����˵ó���9.32�ˣ���ͨ��112mL����״��������ǡ�ý�Fe2+��ȫ�������Ʋ⾧��Ļ�ѧʽΪ
��4�����������[��NH4��2SO4?FeSO4?6H2O]���׳�Ī���Σ������̷��ȶ����ڷ�����ѧ�г���������Fe2+�ı���Һ���ô�Fe2+�ı���Һ���Բⶨʣ��ϡ�����������ȡ8.64��Cu2S��CuS�Ļ������200mL 2mol/Lϡ������Һ������������Ӧ���£�
10NO3-+3Cu2S+16H+=6Cu2++10NO��+3SO42-+8H2O
8NO3-+3CuS+8H+=3Cu2++3SO42-+8NO��+4H2O
ʣ���ϡ����ǡ����V mL 2mol/L ��NH4��2Fe��SO4��2��Һ��ȫ��Ӧ��
��֪��NO3-+3Fe2++4H+=NO��+3Fe3++2H2O
��Vֵ��Χ
����V=48���Լ���������CuS����������
���㣺��ѧ����ʽ���йؼ���
ר�⣺������
��������1������c=
������ܶ�Ϊ1.4g/cm3����������Ϊ50%���������ʵ���Ũ�ȣ������ܶ���ͬ����Ϻ���������Ϊ40%�������������������Խ����Һ�к��е����������Խ�����Ի�Ϻ������������������40%��
��2��20%��������ɱ�ʾΪSO3?nH2O��ͨ��������Ϊ��nH2SO4?��1-n��SO3������20%����������H2SO4��SO3��������ϵ�ɵã�98n��80��1-n��=��1-20%����20%�����n���ɣ�
��3�����ݳ���9.32��Ϊ���ᱵ�����������ʵ�������������Ӻ��������ӵ����ʵ������ٸ���������ϵ��������еĽᾧˮ�������������������Ļ�ѧʽ��
��4���ٲ��ü���������������ﷴӦ��Ҫ����������ʵ�����ʣ��������루NH4��2Fe��SO4��2��Һ��Ӧ������Ҫ�ģ�NH4��2Fe��SO4��2��Һ�������
���ȸ��ݣ�NH4��2Fe��SO4��2�����ʵ����������䷴Ӧ����������ʵ��������������������������������������������������Ҫ����������ʵ�����Ȼ����ʽ��������������������������������ʽ���㼴�ɣ�
| 1000��w |
| M |
��2��20%��������ɱ�ʾΪSO3?nH2O��ͨ��������Ϊ��nH2SO4?��1-n��SO3������20%����������H2SO4��SO3��������ϵ�ɵã�98n��80��1-n��=��1-20%����20%�����n���ɣ�
��3�����ݳ���9.32��Ϊ���ᱵ�����������ʵ�������������Ӻ��������ӵ����ʵ������ٸ���������ϵ��������еĽᾧˮ�������������������Ļ�ѧʽ��
��4���ٲ��ü���������������ﷴӦ��Ҫ����������ʵ�����ʣ��������루NH4��2Fe��SO4��2��Һ��Ӧ������Ҫ�ģ�NH4��2Fe��SO4��2��Һ�������
���ȸ��ݣ�NH4��2Fe��SO4��2�����ʵ����������䷴Ӧ����������ʵ��������������������������������������������������Ҫ����������ʵ�����Ȼ����ʽ��������������������������������ʽ���㼴�ɣ�
���
�⣺��1���ܶ�Ϊ1.4g/cm3����������Ϊ50%���������ʵ���Ũ��Ϊ��
mol/L��7.14mol/L������50%��������30%�������ܶ���ͬ�����Ϻ��������������Ϊ40%������50%��������ܶȴ���30%�����ᣬ���Ի�Ϻ���Һ�����������ƫ�������������������40%��
�ʴ�Ϊ��7.14 mol?L-1������
��2��20%��������ɱ�ʾΪSO3?nH2O��ͨ��������Ϊ��nH2SO4?��1-n��SO3������20%����������H2SO4��SO3��������ϵ�ɵã�98n��80��1-n��=��1-20%����20%�����n=0.77��
�ʴ�Ϊ��0.77��
��3�����ᱵ�����ʵ���Ϊ��
=0.04mol����n��FeSO4��+3n��Fe2��SO4��3��=0.04mol�������112mL���������ʵ���Ϊ��
=0.005mol�����ݷ�Ӧ��ϵʽ2Fe2+��Cl2��֪���������������ʵ���Ϊ��n��FeSO4��=n��Fe2+��=2n��Cl2��0.01mol����3n��Fe2��SO4��3��=0.04mol-0.01mol=0.03mol��7.32�˾����нᾧˮ������Ϊ��m��H2O��=7.32g-152g/mol��0.01mol-400g/mol��0.01mol=1.8g���ᾧˮ�����ʵ���Ϊ��n��H2O��=
=0.1mol�������̷���������������������ͭ��ˮ�����ʵ���֮��Ϊ0.01mol��0.01mol��0.1mol=1��1��10�����Ծ���Ļ�ѧʽΪ��FeSO4?Fe2��SO4��3?10H2O��
�ʴ�Ϊ��FeSO4?Fe2��SO4��3?10H2O��
��4������������ʵ���Ϊ��0.20L��2mol/L=0.4mol��
�����������ȫ��ΪCu2S����n��Cu2S��=
=0.054mol����Ҫ����������ʵ���Ϊx��
10NO3-+3Cu2S+16H+��6Cu2++10NO��+3SO42-+8H2O
3 16
0.054mol x
x=
=0.288mol��
ʣ�����������ʵ���Ϊ��0.4mol-0.288mol=0.112mol��
0.112mol����ͣ�NH4��2Fe��SO4��2��Һ��ȫ��Ӧ��
NO3-+3Fe2++4H+��NO��+3Fe3++2H2O
3mol 4
1��10-3VL��2mol/L 0.112mol
���V=42��
�����������ȫ��ΪCuS����n��CuS��=
=0.09mol����Ҫ��������ʵ���Ϊy��
8NO3-+3CuS+8H+��3Cu2++3SO42-+8NO��+4H2O��
3 8
0.09mol y
y=
=0.24mol
ʣ�����������ʵ���Ϊ��0.4mol-0.24mol=0.16mol��
0.112mol����ͣ�NH4��2Fe��SO4��2��Һ��ȫ��Ӧ��
NO3-+3Fe2++4H+��NO��+3Fe3++2H2O
3mol 4
1��10-3VL��2mol/L 0.16mol
���V=60��
����Vֵ��ΧΪ��42��V��60��
�ʴ�Ϊ��42��60mL��
����V=48����48mL��NH4��2Fe��SO4��2��Һ��ȫ��Ӧ��Ҫ��������ʵ���Ϊ��
NO3-+3Fe2++4H+��NO��+3Fe3++2H2O
3mol 4
0.048L��2mol/L n
��ã�n=0.128mol��
�������ĵ���������ʵ���Ϊ0.128mol��
����������ﷴӦ����������ʵ���Ϊ��0.4mol-0.128mol=0.272mol��
��Cu2S�����ʵ���xmol��CuS�����ʵ���Ϊymol��160x+96y=8.64g�٣�
10NO3-+3Cu2S+16H+=6Cu2++10NO��+3SO42-+8H2O
3 16
x
8NO3-+3CuS+8H+��3Cu2++3SO42-+8NO��+4H2O��
3 8
y
x+
y=0.272��
�ɢ٢ڽ�ã�
��
������CuS������������
��0.33��
�ʴ�Ϊ��0.33��
| 1000��1.4��50% |
| 98 |
�ʴ�Ϊ��7.14 mol?L-1������
��2��20%��������ɱ�ʾΪSO3?nH2O��ͨ��������Ϊ��nH2SO4?��1-n��SO3������20%����������H2SO4��SO3��������ϵ�ɵã�98n��80��1-n��=��1-20%����20%�����n=0.77��
�ʴ�Ϊ��0.77��
��3�����ᱵ�����ʵ���Ϊ��
| 9.32g |
| 233g/mol |
| 112��10-3 |
| 22.4L/mol |
| 1.8g |
| 18g/mol |
�ʴ�Ϊ��FeSO4?Fe2��SO4��3?10H2O��
��4������������ʵ���Ϊ��0.20L��2mol/L=0.4mol��
�����������ȫ��ΪCu2S����n��Cu2S��=
| 8.64g |
| 160g/mol |
10NO3-+3Cu2S+16H+��6Cu2++10NO��+3SO42-+8H2O
3 16
0.054mol x
x=
| 0.054mol��16 |
| 3 |
ʣ�����������ʵ���Ϊ��0.4mol-0.288mol=0.112mol��
0.112mol����ͣ�NH4��2Fe��SO4��2��Һ��ȫ��Ӧ��
NO3-+3Fe2++4H+��NO��+3Fe3++2H2O
3mol 4
1��10-3VL��2mol/L 0.112mol
���V=42��
�����������ȫ��ΪCuS����n��CuS��=
| 8.64g |
| 96g/mol |
8NO3-+3CuS+8H+��3Cu2++3SO42-+8NO��+4H2O��
3 8
0.09mol y
y=
| 0.09mol��8 |
| 3 |
ʣ�����������ʵ���Ϊ��0.4mol-0.24mol=0.16mol��
0.112mol����ͣ�NH4��2Fe��SO4��2��Һ��ȫ��Ӧ��
NO3-+3Fe2++4H+��NO��+3Fe3++2H2O
3mol 4
1��10-3VL��2mol/L 0.16mol
���V=60��
����Vֵ��ΧΪ��42��V��60��
�ʴ�Ϊ��42��60mL��
����V=48����48mL��NH4��2Fe��SO4��2��Һ��ȫ��Ӧ��Ҫ��������ʵ���Ϊ��
NO3-+3Fe2++4H+��NO��+3Fe3++2H2O
3mol 4
0.048L��2mol/L n
��ã�n=0.128mol��
�������ĵ���������ʵ���Ϊ0.128mol��
����������ﷴӦ����������ʵ���Ϊ��0.4mol-0.128mol=0.272mol��
��Cu2S�����ʵ���xmol��CuS�����ʵ���Ϊymol��160x+96y=8.64g�٣�
10NO3-+3Cu2S+16H+=6Cu2++10NO��+3SO42-+8H2O
3 16
x
| 16x |
| 3 |
8NO3-+3CuS+8H+��3Cu2++3SO42-+8NO��+4H2O��
3 8
y
| 8y |
| 3 |
| 16 |
| 3 |
| 8 |
| 3 |
�ɢ٢ڽ�ã�
|
������CuS������������
| 96g/mol��0.03mol |
| 8.64g |
�ʴ�Ϊ��0.33��
���������⿼�������ʵ���Ũ�ȵļ��㡢���ӻ�ѧʽ��ȷ������ѧ����ʽ�ļ����֪ʶ����Ŀ�ѶȽϴ��漰�ļ������Դ�ע���������ʵ����������ʽ����ȷ���ݻ�ѧ����ʽ���м���ķ��������У�4��Ϊ�ѵ㣬�漰���������۷����⣮

��ϰ��ϵ�д�
 ������ҵ��ͬ����ϰ��ϵ�д�
������ҵ��ͬ����ϰ��ϵ�д�
�����Ŀ
����ͼʾ���Ӧ��������������ǣ�������
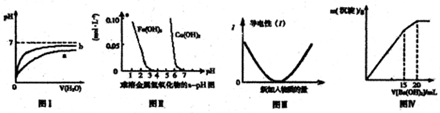

| A��ͼ���ʾpH��ͬ������������зֱ����ˮ����ҺpH�ı仯����������b��Ӧ�������� |
| B������ͼ���֪������ȥCuSO4��Һ�е�Fe3+��������Һ�м���CuO2����pH��4-5֮�伴�� |
| C��ͼ��ɱ�ʾ������Һ��ͨ�백���������Ĺ�������Һ�����Եı仯 |
| D��ͼ����ʾij������Һ�м���Ba��OH��2��Һ�����������������Ba��OH��2��Һ����Ĺ�ϵ���ڼ���20mLBa��OH��2��Һʱ������ȫ����BaSO4 |
C��CuO��һ���¶��·�Ӧ��������Cu��Cu2O��CO��CO2������2.00g C��16.0g CuO��ϣ������������ȣ������ɵ�����ȫ��ͨ�������ij���ʯ��ˮ����Ӧһ��ʱ����ռ���1.12L���壨��״���������ɳ���������Ϊ5.00g������˵��������ǣ�������
| A����Ӧ��Ĺ���������Cu������Ϊ12.8 g |
| B����Ӧ��Ĺ��������л�����̼ |
| C����Ӧ��Ĺ�������������Ϊ13.6 g |
| D����Ӧ��Ĺ�������������������ʵ���Ϊ0.05mol |
������100mL 1.0mol/L��Na2SO4��Һ�����в�����ȫ��ȷ���ǣ�������
�ٽ�14.2g Na2SO4����100mL����ˮ��
����������ƽ����32.20g�� Na2SO4?10H2O���壬������������ˮ�У���ϡ����100mL
�۽�20mL5.0mol/L Na2SO4��Һ������ˮϡ����100mL��
�ٽ�14.2g Na2SO4����100mL����ˮ��
����������ƽ����32.20g�� Na2SO4?10H2O���壬������������ˮ�У���ϡ����100mL
�۽�20mL5.0mol/L Na2SO4��Һ������ˮϡ����100mL��
| A���� | B���ڢ� | C���٢� | D���٢� |
��������������ǣ�������
| A����ϩ�ͱ�����ʹ��ˮ����ɫ��ȥ����ɫ��ԭ����ͬ |
| B�����ۡ���֬�������ʶ���ˮ�⣬��ˮ����ﲻͬ |
| C��ú�Ϳ���ʯ�ͷ����ã�������ȼ�Ϻͱ������������� |
| D���Ҵ������ᡢ�����������ܷ���ȡ����Ӧ�����������е�����������ñ���Na2CO3��Һ��ȥ |
������ʵ����˵������Ϊ������ǣ�������
| A��ϡ������Һ�ĵ����ԱȽ��� |
| B����CH3COONa��Һ�е����̪��Һ����Һ���ɫ |
| C��һ���¶��£���ij������Һ�м���CH3COONH4���壬��ҺpH���� |
| D��һ���¶��£��������ͬ��pHֵҲ��ͬ������ʹ����У�Ͷ����ͬ������п����п����ȫ�ܽ������ĵ�ʱ�䲻ͬ |
����˵�����ʾ��������ǣ�������
| A����ѧ��Ӧ�����е������仯���������⣬�������ǹ��ܡ����ܵ� |
| B�����ȷ�Ӧ�ġ�H��0 |
| C����Ҫ���Ȳ��ܷ�����Ӧһ�������ȷ�Ӧ |
| D���Ȼ�ѧ����ʽ�еĻ�ѧ����������ʾ�����ʵ����ʵ����������Ƿ��� |
 ���ִ�ս���У�����̹��ս����õ�װ�ײ����Ǿ������ƺ��ȴ�����ĺϽ�֣��ȴ���������װ�ṹ�Ļ�ѧ�ͻ�е���Ժ�����ȵı���һ�£����кϽ�Ԫ�صİٷֱȺ���Ϊ����0.5��1.25 ��0.5��1.5 ��0.3��0.6 ��0.8��1.6 ̼0.3
���ִ�ս���У�����̹��ս����õ�װ�ײ����Ǿ������ƺ��ȴ�����ĺϽ�֣��ȴ���������װ�ṹ�Ļ�ѧ�ͻ�е���Ժ�����ȵı���һ�£����кϽ�Ԫ�صİٷֱȺ���Ϊ����0.5��1.25 ��0.5��1.5 ��0.3��0.6 ��0.8��1.6 ̼0.3