��Ŀ����
7����֪һ��̼ԭ�������������ǻ�ʱ����������ת����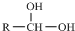 $\stackrel{-H_{2}O}{��}$
$\stackrel{-H_{2}O}{��}$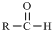 �������ͼ�ش�
�������ͼ�ش�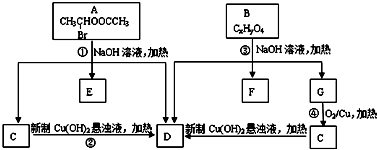
��1��A�����������ŵ�����Ϊ��������ԭ�ӣ�
��2������������B������ʺɱ�Ϊ208�����������ʾB�����к��б����ṹ�������������˴Ź�����������������շ壬���ֵ��Ϊ2��2��2��3��3�����б����ϵ�һ�ȴ���ֻ�����֣���B�Ľṹ��ʽΪ
 ��
����3��д�����з�Ӧ����ʽ��
��
 ��
����
 ��
����4����������������B��ͬ���칹�干��9�֣�
�����ڷ����廯���
�ں�������ȡ����������ֻ��һ��������������ȡ������ͬ�Ҵ�������λ�ã�
���ܷ���ˮ�ⷴӦ��������Ӧ��
��5����֪��2RCH2COOC2H5$\stackrel{Na}{��}$
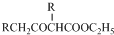 +C2H5OH������GΪΨһ�л��Լ��ϳ���������������CH3COCH2COOC2H5������ƺϳ�·�ߣ������Լ���ѡ����
+C2H5OH������GΪΨһ�л��Լ��ϳ���������������CH3COCH2COOC2H5������ƺϳ�·�ߣ������Լ���ѡ�����ϳ�·������ͼʾ����CH3CH2Cl$��_{��}^{NaOH��Һ}$CH3CH2OH$��_{Ũ���ᡢ��}^{CH_{3}COOH}$CH3COOC2H5��
���� A����ˮ�ⷴӦC��D��E��C������D������������Ϣ��֪��C�Ľṹ��ʽΪCH3CHO��DΪCH3COONa��EΪNaBr��B������ʺɱ�Ϊ208������B����Է�������Ϊ208�����������ʾB�����к��б����ṹ�������������˴Ź�����������������շ壬���ֵ��Ϊ2��2��2��3��3�����б����ϵ�һ�ȴ���ֻ�����֣�B����ˮ���D��G��F��G���������õ�D����GΪCH3CH2OH������֪BΪ ��B����ˮ���FΪ
��B����ˮ���FΪ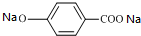 ��
��
��5��GΪCH3CH2OH����CH3CH2OHΪԭ�Ϻϳ���������������CH3COCH2COOC2H5�������������Ҵ����������ᣬ�Ҵ����Ҵ����������������������������Ƶ������·���������Ϣ�еķ�Ӧ����������������
��� �⣺��1������A�Ľṹ��ʽ��֪��A�����������ŵ�����Ϊ��������ԭ�ӣ�
�ʴ�Ϊ����������ԭ�ӣ�
��2����������ķ�����֪��B�Ľṹ��ʽΪ ��
��
�ʴ�Ϊ�� ��
��
��3����Ӧ�ٵķ���ʽΪ ��
��
��Ӧ �ܵķ���ʽΪ ��
��
�ʴ�Ϊ�� ��
�� ��
��
��4��BΪ ����ͬ���칹����ϣ������ڷ����廯���˵���б������ں�������ȡ����������ֻ��һ��������������ȡ������ͬ�Ҵ�������λ�ã����ܷ���ˮ�ⷴӦ��������Ӧ��˵����������ȩ���������������B��ͬ���칹��Ϊ�ڱ����ļ�λ��������-OOCH��һ��-C3H7�����������з�������-C3H7��2�ֽṹ�����Թ���6�ֽṹ��Ҳ�������ڱ����ļ�λ��������HCOOCH2-��һ��-CH3�����������з��������Թ���9�֣�
����ͬ���칹����ϣ������ڷ����廯���˵���б������ں�������ȡ����������ֻ��һ��������������ȡ������ͬ�Ҵ�������λ�ã����ܷ���ˮ�ⷴӦ��������Ӧ��˵����������ȩ���������������B��ͬ���칹��Ϊ�ڱ����ļ�λ��������-OOCH��һ��-C3H7�����������з�������-C3H7��2�ֽṹ�����Թ���6�ֽṹ��Ҳ�������ڱ����ļ�λ��������HCOOCH2-��һ��-CH3�����������з��������Թ���9�֣�
�ʴ�Ϊ��9��
��5����CH3CH2OHΪԭ�Ϻϳ���������������CH3COCH2COOC2H5�������������Ҵ����������ᣬ�Ҵ����Ҵ����������������������������Ƶ������·���������Ϣ�еķ�Ӧ�����������������ϳ�·��Ϊ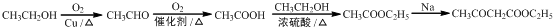 ��
��
�ʴ�Ϊ��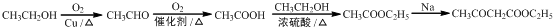 ��
��
���� ���⿼���л����ƶ���ϳɣ�Ϊ�߿��������ͣ����ؿ���ѧ�������ƶ�������ȷ��B�Ľṹ��ʽ�ǽⱾ��ؼ��������л���Ĺ����ŵı仯Ϊͻ�ƿڽ����ƶϣ���Ҫѧ���������չ����ŵĽṹ�����ʣ��ѵ���ͬ���칹�������жϣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | ���� | B�� | �� | C�� | �Ȼ�����Һ | D�� | CuSO4��Һ |
| A�� | ����NH4+��Cl-��H+��OH-����Һ�У�������Ũ��һ���ǣ�c��Cl-����c��NH4+����c��H+����c��OH-�� | |
| B�� | pH=6�Ĵ���������ƵĻ����Һ�У�c��Na+����c��CH3COO-�� | |
| C�� | 0.1 mol/L ��Na2S��Һ�У�c��OH��=c��H+��+c��HS-��+2c��H2S�� | |
| D�� | pH=3��һԪ���pH=11��һԪ��������ͺ����Һ�У�һ����c��OH-��=c��H+�� |
| A�� | 25��ʱ1mLˮ�к�10-10 NA��OH-���� | |
| B�� | 1molCl2ͨ��������NaOH��Һ�г�ַ�Ӧת�Ƶĵ�����Ϊ2NA | |
| C�� | a gij���庬������Ϊb��c g�������ڱ���µ����ԼΪ22.4bc/��aNA��L | |
| D�� | 0.1mol�ƺ�O2��һ�������·�Ӧ����Na2O��Na2O2�����ʱ��ʧȥ������Ϊ0.1NA |

| A�� | ��ʼʱ��ҺpH=2����Ϊ����Һ�л�������ʣ�� | |
| B�� | BC�α�ʾ����̼������Һ�ĵ��룬CaCO3���������������� | |
| C�� | AB�����ķ�ӦΪ��Ca2++CO32-�TCaCO3�� | |
| D�� | ����500��̼������Һ����Һ��c��OH-����c��H+�� |
| A�� | NaHCO3��NaOH��Һ��Ӧ��HCO3-+OH-�TCO32-+H2O | |
| B�� | ������ˮ��Ӧ��Cl2+H2O?2H++Cl��+ClO�� | |
| C�� | �Ȼ�����Һ�м��������İ�ˮ��Fe3++3NH3•H2O=Fe��OH��3��+3NH4+ | |
| D�� | ʢ�ż����Լ�����Һ�����ò�������ԭ��SiO2+2OH-�TSiO32-+H2O |

| A�� | ������е���ȡ����Ϊ�����仯 | |
| B�� | ����۷����ķ�Ӧ��I2����������NaOH�ǻ�ԭ�� | |
| C�� | ��������ӵ��Լ�X������ϡ���� | |
| D�� | ������漰����Ҫ����YΪ��Һ������ |
 ����������˵����ȷ���ǣ�������
����������˵����ȷ���ǣ�������| A�� | ������Ļ�ѧʽΪ��C13H14O4Cl2 | |
| B�� | ������������4 molH2������Ӧ | |
| C�� | �������ʹ������Ȼ�̼��Һ��ɫ | |
| D�� | ��������һ���������ܷ�����ȥ��Ӧ |
| A�� | 1mol S��g����1mol O2��g������������1mol SO2��g������������Q kJ | |
| B�� | 1mol S��g����1mol O2��g����Ӧ����1mol SO2��g���ų�Q kJ������ | |
| C�� | S��s��+O2��g��=SO2��g����H��-Q kJ•mol-1 | |
| D�� | 1��S��g����1��O2��g����ȫ��Ӧ���Էų�Q kJ������ |