��Ŀ����
18��������Һ�У���Ũ�ȹ�ϵ��ȷ���ǣ�������| A�� | ����NH4+��Cl-��H+��OH-����Һ�У�������Ũ��һ���ǣ�c��Cl-����c��NH4+����c��H+����c��OH-�� | |
| B�� | pH=6�Ĵ���������ƵĻ����Һ�У�c��Na+����c��CH3COO-�� | |
| C�� | 0.1 mol/L ��Na2S��Һ�У�c��OH��=c��H+��+c��HS-��+2c��H2S�� | |
| D�� | pH=3��һԪ���pH=11��һԪ��������ͺ����Һ�У�һ����c��OH-��=c��H+�� |
���� A������NH4+��Cl-��H+��OH-����Һ�������غ㼴�ɣ�
B��pH=6�Ĵ���ʹ����ƵĻ����Һ�����ԣ���c��H+����c��OH-����
C�����������غ���������
D��pH=3�����pH=11��һԪ���ǿ��δ֪��
��� �⣺A������NH4+��Cl-��H+��OH-����Һ�������غ㼴�ɣ�������Ũ�ȿ���Ϊc��Cl-����c��NH4+����c��H+����c��OH-����������Ϊc��OH-����c��NH4+����c��H+����c��Cl-��������c��Cl-����c��H+����c��NH4+����c��OH-������A����
B��pH=6�Ĵ���ʹ����ƵĻ����Һ�����ԣ���c��H+����c��OH-�������ݵ���غ��֪��c��Na+����c��CH3COO-����B����
C��0.1 mol/L ��Na2S��Һ�У�S2-���ˮ������������Ӷ�ˮ��ΪHS-��H2S������ˮ������������ӵĴ�����ʽ�����֣�H+��HS-��H2S���ʸ��������غ��֪��c��OH��=c��H+��+c��HS-��+2c��H2S������C��ȷ��
D��pH=3�����pH=11��һԪ���ǿ��δ֪��������Ϻ�ģ���������������ܼ������������ǡ����ȫ��Ӧ���ʻ�Ϻ���Һ�������δ֪����D����
��ѡC��
���� ���⿼��������ˮ�⣬������Һ������ٽ�ϵ���غ�����������ѵ��������غ�����ú����⣮

 ����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�
����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�| A�� | FeCl3 | B�� | Na2S | C�� | ��NH4��2CO3 | D�� | Na2SO4 |
| A�� | CH4 | B�� | CO | C�� | NO2 | D�� | Na2O |
| ѡ�� | ʵ����� | ���� | ���� |
| A | ���ܱ������м���CuO��1000�� | ��ɫ�����ɺ�ɫ���� | CuO���ȷֽ�õ�����Cu |
| B | ��SO2ͨ��Ʒ����Һ�� | ��Һ��ɫ | SO2����Ư���� |
| C | ��Mg��Al��NaOH��Һ���ԭ��� | Al�缫�ܽ� | Al��Mg�������ǿ |
| D | ��ij��Һ�м��������ữ���Ȼ�����Һ | �а�ɫ�������� | ����Һ��һ������SO42- |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ���³�ѹ�£�3.2 g O3����������Ϊ1.2 NA | |
| B�� | ��״���£�2.24 L CCl4�к��е�C-Cl������ĿΪ0.4 NA | |
| C�� | CO��N2��Ϊ�ȵ����壬��״����11.2 L CO��0.5 molN2������������� | |
| D�� | ��0.1 mol �Ȼ�������1 Lˮ�У�������Һ����0.1 NA Fe3+ |
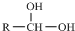 $\stackrel{-H_{2}O}{��}$
$\stackrel{-H_{2}O}{��}$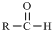 �������ͼ�ش�
�������ͼ�ش�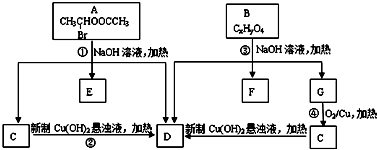
 ��
�� ��
�� ��
��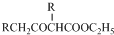 +C2H5OH������GΪΨһ�л��Լ��ϳ���������������CH3COCH2COOC2H5������ƺϳ�·�ߣ������Լ���ѡ����
+C2H5OH������GΪΨһ�л��Լ��ϳ���������������CH3COCH2COOC2H5������ƺϳ�·�ߣ������Լ���ѡ����