��Ŀ����
6��25��ʱ����ʢ��50mLpH=2��һԪ��HA��Һ�ľ��������м���pH=13��NaOH��Һ������NaOH��Һ�������V�������û����Һ���¶ȣ�T���Ĺ�ϵ��ͼ��ʾ������������ȷ���ǣ�������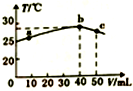
| A�� | 25��ʱ��HA�ĵ���ƽ�ⳣ��KaԼΪ1.43��10-3 | |
| B�� | a��b�Ĺ����У���Һ��c��A-����c��HA��֮��ʼ�ղ��� | |
| C�� | b��c�Ĺ����У��¶Ƚ��͵���Ҫԭ������Һ�з��������ȷ�Ӧ | |
| D�� | ��Ũ�ȵ�NaOH��NaA�����Һ��һ�����ڹ�ϵ��c��Na+����c��A-����c��OH-����c��H+�� |
���� A������ƽ�ⳣ��K=$\frac{c��{H}^{+}��c��{A}^{-}��}{c��HA��}$����õ��жϣ�
B��������к�ǡ����ȫʱ����Һ�е�����ΪNaA�����������غ�c��A-����c��HA��֮��ʼ�յ���������Ũ�ȣ��������������ʱ������ȣ�
C��b��c�Ĺ����У��¶Ƚ��͵�ԭ������Һ�з����˷�Ӧǡ������NaA�������μ�����������Һ���ٷ�����Ӧ��
D�����������غ㣬��Ũ�ȵ�NaOH��NaA�����Һ��c��Na+����c��OH-����c��A-����c��H+����
��� �⣺A������ƽ�ⳣ��K=$\frac{c��{H}^{+}��c��{A}^{-}��}{c��HA��}$=$\frac{0.01mol/L��0.01mol/L}{0.08mol/L-0.01mol/L}$=1.43��10-3����A��ȷ��
B��������к�ǡ����ȫʱ����Һ�е�����ΪNaA�����������غ�c��A-��+c��HA��=c��Na+�����������������ʱ������ȣ���B����
C��b��c�Ĺ����У��¶Ƚ��͵�ԭ������Һ�з����˷�Ӧǡ������NaA�������μ�����������Һ���ٷ�����Ӧ����Һ�¶Ƚ��ͣ���C����
D�����������غ㣬��Ũ�ȵ�NaOH��NaA�����Һ��c��Na+����c��OH-����c��A-����c��H+������D����
��ѡA��
���� ���⿼������ϵĶ����жϺͼ��㣬��Ŀ����кͷ�Ӧ������ͬѧ�ǹ۲����������������Լ����û�ѧ����ʽ������������Ƚ��ۺϣ�

 �����ÿ�ʱѵ��ϵ�д�
�����ÿ�ʱѵ��ϵ�д� ��Ԫȫ��������ϵ�д�
��Ԫȫ��������ϵ�д� �»ƸԱ����ܾ�ϵ�д�
�»ƸԱ����ܾ�ϵ�д�| A�� | ������Һ�ĵ����Ա�������Һ���� | |
| B�� | 0.1mol/L��������Һ��pHԼΪ8 | |
| C�� | ��0.1mol/L������Һ�¶�����10�������ˮ���������� pH���� | |
| D�� | ��Ũ�ȵ����������������ڿ�ʼ��ͬ����С��þ��Ӧʱ�����ᷴӦ�� |
| ���� | һ�ȴ��� | |
| A | 2-��-2-�һ����� | 3 |
| B | 1��3-������ | 3 |
| C | 2��2��3-�������� | 6 |
| D | 2��3-����-4-�һ����� | 7 |
| A�� | A | B�� | B | C�� | C | D�� | D |
| ���� | CH3COOH | HCN | H2CO3 |
| ����ƽ�ⳣ����25�棩 | K1=1.8��10-5 | K1=4.9��10-10 | K1=4.3��10-7 K2=5.6��10-11 |
| A�� | �����ʵ���Ũ��ʱ��pH��Na2CO3����pH��NaCN����pH��NaHCO3����pH��CH3COONa�� | |
| B�� | �к͵��������pH��CH3COOH��Һ��HCN��Һ����NaOH����ǰ�ߴ��ں��� | |
| C�� | ��NaCN��ͨ��������CO2��CN -+H2O+CO2=HCO3 -+HCN | |
| D�� | 0.2mol/L HCN��0.1mol/L NaOH��Һ�������Ϻ��Լ��ԣ���c��Na+����c��CN-�� |
| ѡ�� | ��ѧ��Ӧ | �������ݣ���λʱ���ڣ� |
| A | CO��g��+H2O��g��=CO2��g��+H2��g�� | ѹǿ�仯 |
| B | Zn+H2SO4=ZnSO4+H2 | H2��� |
| C | 2NO2?N2O4 | ��ɫ��dz |
| D | Ca��OH��2+Na2CO3=CaCO3��+2NaOH | �������� |
| A�� | A | B�� | B | C�� | C | D�� | D |
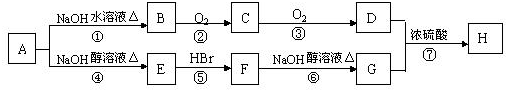
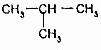 �Ĺ�ϵ��ͬϵ��
�Ĺ�ϵ��ͬϵ��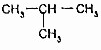 ���춡�飩�Ĺ�ϵ��ͬ���칹�壮
���춡�飩�Ĺ�ϵ��ͬ���칹�壮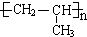 ��
�� ����ԭ�ӽṹ�ĽǶȷ���B��N��O�ĵ�һ�������ɴ�С��˳��ΪN��O��B��
����ԭ�ӽṹ�ĽǶȷ���B��N��O�ĵ�һ�������ɴ�С��˳��ΪN��O��B��