��Ŀ����
15�������£���������ĵ���ƽ�ⳣ�������| ���� | CH3COOH | HCN | H2CO3 |
| ����ƽ�ⳣ����25�棩 | K1=1.8��10-5 | K1=4.9��10-10 | K1=4.3��10-7 K2=5.6��10-11 |
| A�� | �����ʵ���Ũ��ʱ��pH��Na2CO3����pH��NaCN����pH��NaHCO3����pH��CH3COONa�� | |
| B�� | �к͵��������pH��CH3COOH��Һ��HCN��Һ����NaOH����ǰ�ߴ��ں��� | |
| C�� | ��NaCN��ͨ��������CO2��CN -+H2O+CO2=HCO3 -+HCN | |
| D�� | 0.2mol/L HCN��0.1mol/L NaOH��Һ�������Ϻ��Լ��ԣ���c��Na+����c��CN-�� |
���� A��ͼ���е���ƽ�ⳣ����֪����Һ����CH3COOH��H2CO3��HCN��HCO3-����Խ����Ӧ��ˮ��̶�Խ����Һ����Խǿ��
B����pH��HCOOH��HCN��Һ����������ʵ���Ũ��С�������
C����Һ����CH3COOH��H2CO3��HCN��HCO3-����NaCN��ͨ��������CO2��ֻ������̼�������
D.0.2mol/L HCN��0.1mol/L NaOH��Һ�������Ϻ��Լ��ԣ�������Һ�е���غ�����жϣ�
��� �⣺A��ͼ���е���ƽ�ⳣ����֪����Һ����CH3COOH��H2CO3��HCN��HCO3-�������ʵ���Ũ��ʱ��pH��Na2CO3����pH��NaCN����pH��NaHCO3����pH��CH3COONa������A��ȷ��
B����pH��HCOOH��HCN��Һ����������ʵ���Ũ��С�������ᣬ�����к͵��������pH��CH3COOH��HCN����NaOH����ǰ��С�ں��ߣ���B����
C����Һ����CH3COOH��H2CO3��HCN��HCO3-����NaCN��ͨ��������CO2��ֻ������̼����������ӷ���ʽΪ��CN -+H2O+CO2=HCO3 -+HCN����C��ȷ��
D.0.2mol/L HCN��0.1mol/L NaOH��Һ�������Ϻ��Լ��ԣ�c��H+����c��OH-������Һ���ڵ���غ㣺c��Na+��+c��H+��=c��OH-��+c��CN-����c��Na+����c��CN-������D��ȷ��
��ѡB��
���� ���⿼����������ʵ���ƽ�ⳣ���Ƚϡ�����ˮ�������ע����Һ�е���غ��������ʵ���ƽ���Ӱ�����ص�����Ӧ�ã����ջ����ǽ���ؼ�����Ŀ�Ѷ��еȣ�

| A�� | �ܢݢޢ� | B�� | �ܢݢ� | C�� | �ڢܢ� | D�� | �ڢܢݢ� |
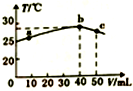
| A�� | 25��ʱ��HA�ĵ���ƽ�ⳣ��KaԼΪ1.43��10-3 | |
| B�� | a��b�Ĺ����У���Һ��c��A-����c��HA��֮��ʼ�ղ��� | |
| C�� | b��c�Ĺ����У��¶Ƚ��͵���Ҫԭ������Һ�з��������ȷ�Ӧ | |
| D�� | ��Ũ�ȵ�NaOH��NaA�����Һ��һ�����ڹ�ϵ��c��Na+����c��A-����c��OH-����c��H+�� |
| A�� | X��ԭ��������Y��С | B�� | X��ԭ�Ӱ뾶��Y�Ĵ� | ||
| C�� | Xԭ�ӵ�������������Y�Ĵ� | D�� | XԪ�ص�������۱�Y�Ĵ� |
| A�� | Ba��OH��2•8H2O��NH4Cl��Ӧ | B�� | ���ȵ�C��CO2�ķ�Ӧ | ||
| C�� | CH4��O2��ȼ�շ�Ӧ | D�� | ����ϡ����ķ�Ӧ |
| A�� | ����ĵ���ʽ | B�� | ̼-12ԭ�ӣ�612C | ||
| C�� | �����Ƶĵ���ʽ | D�� | �����ӵĽṹʾ��ͼ |
| A�� | 92235Uԭ�Ӻ��к���92������ | B�� | 92235U�Ӻ�����143������ | ||
| C�� | 92235U��92238U��Ϊͬλ�� | D�� | 92235U��92238U��Ϊͬ�������� |