��Ŀ����
20��Ĥ����ԭ���ڻ������������Ź㷺��Ӧ�ã������������õ绯ѧԭ���Ʊ������������ɫ������N2O5��װ��ͼ���£�����˵������ȷ���ǣ�������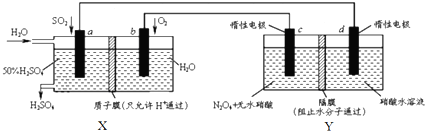
| A�� | X��ԭ��أ��ܹ��������ᣮY�ǵ��أ��ܹ�����N2O5 | |
| B�� | c�缫�ĵ缫��Ӧ����ʽΪ��N2O4+2HNO3-2e-=2N2O5+2H+ | |
| C�� | ����·��ͨ��2mol e-��X��Y�и���1molH+�����Ǩ�Ƶ��ұ� | |
| D�� | Ϊ��֤X������������������䣬������n��so2����n��H2O��=1��7.4 |
���� ��A����ͼ��֪��Xװ�����Է��Ľ���������ԭ��Ӧ��û����ӵ�Դ������X��ԭ��أ��ܷ�ӦΪ2SO2+2H2O+O2=2H2SO4�����Բ������ᣬ��������ĵ缫ʧ���ӷ���������Ӧ��Ϊ��������bΪ������Y���ڵ��أ����Դ������b�����ĵ缫cΪ������N2O4������ʧ��������N2O5��
��B��c��������d��������������N2O4�ŵ�����N2O5��
��C�����ݵ缫��Ӧʽ�ó�ת�Ƶ��ӵ����ʵ����������ӵĹ�ϵ���ٴ���������Һ�е��ƶ������жϣ�
��D��Xװ���У�SO2-2e-+2H2O=SO42-+4H+�����ݹ�ϵʽ���㣬ע�������Ũ�Ȳ��䣮
��� �⣺��A��Xװ�����Է��Ľ���������ԭ��Ӧ��û����ӵ�Դ������X��ԭ��أ��ܷ�ӦΪ2SO2+2H2O+O2=2H2SO4�����Բ������ᣬͨ���������ĵ缫ʧ���ӷ���������Ӧ��Ϊ��������bΪ������Y���ڵ��أ����Դ������b�����ĵ缫cΪ������N2O4������ʧ��������N2O5����A��ȷ��
��B��c��������d��������������N2O4�ŵ�����N2O5���缫��ӦΪN2O4-2e-+2HNO3=2N2O5+2H+����B��ȷ��
��C��Xװ����SO2-2e-+2H2O=SO42-+4H+�����й�ϵʽ��SO2��2e-��4H+��Y��N2O4-2e-+2HNO3=2N2O5+2H+�����й�ϵʽ��N2O4��2e-��2H+�����Ե����������ӵĹ�ϵǰ��Ϊ1��2������Ϊ1��1������·��ͨ��2mol e-��X�и�����4molH+�������������������ƶ����������Ǩ�Ƶ��ұߣ�Y��������2molH+�������������������ƶ����������Ǩ�Ƶ��ұߣ���C����
��D��Xװ���У�SO2-2e-+2H2O=SO42-+4H+������1molSO2��Ӧ������ˮ2mol���������������Ϊ��1mol��98g/mol=98g������ˮ������Ϊ��2mol��18g/mol=36g����ͨ��xmolH2O�����У�$\frac{98g}{98g+x��18g•mo{l}^{-1}-36g}$��100%=50%�����x=7.4��������n��so2����n��H2O��=1��7.4����D��ȷ��
��ѡC��
���� ���⿼����ԭ��غ͵���ԭ���������Ƿ��Է������ж�ԭ��غ͵��أ��ٽ�ϸ����缫�Ϸ����ĵ缫��Ӧ��������ѵ��ǵ缫��Ӧʽ����д�����ļ�������׳�����Ҫע������Ĥֻ����������ͨ������ˮ���Ӳ���ͨ�������Բ������ܷ�Ӧʽ���⣬����õ��ı�����ϵ��1��6.4����Ŀ��һ�����Ѷȣ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
H2��g��+Cl2��g���T2HCl��g����Ӧ�������仯ʾ��ͼ
����˵����ȷ���ǣ�������
| A�� | �Ȼ�����ӵĵ���ʽ�� | |
| B�� | �÷�Ӧ�ǹ�ҵ��ȡ����Ļ�ѧ��Ӧԭ�� | |
| C�� | �γ�1molH-Cl��Ҫ����431 kJ������ | |
| D�� | �÷�Ӧ�з�Ӧ��������С�������������� |
| A�� | ���ʵķе㣺Z��X��Y | |
| B�� | ���ʵ������ԣ�W��Z��Y��X | |
| C�� | ��̬�⻯����ȶ��ԣ�W��X��Y��Z | |
| D�� | W���ʿ��Խ�X��������Һ���û����� |
 ������Ԫ�ؼס����ԭ��������������ϱ�����Ϣ���ش��й����⣮
������Ԫ�ؼס����ԭ��������������ϱ�����Ϣ���ش��й����⣮| �� | �� | �� | �� | �� | |
| ��Ҫ���ϼ� | +1��-1 | +4��-4 | |||
| ���ʻ�ṹ��Ϣ | ͬλ����3�� | ͬ���������ж��� | 2s22p4 | ����ԭ����ԭ�Ӱ뾶���δ�ɶԵ�����Ϊ0 | �����ֳ������������һ���Ǵ�����Ⱦ�� |
��2����Ԫ������ͬ������������Ԫ��ԭ�ӵĵ�һ�������ɴ�С��˳��ΪMg��Al��Na����Ԫ�ط��ű�ʾ�����Խ���ԭ��Mg�����Ų�3sȫ���ṹ����һ�������쳣����
��3���ɼ��ұ����ױ�����ɵ��������ӵ����ο��Է�Ӧ�����ӷ���ʽ��HCO3-+HSO3-=H2O+CO2��+SO32-��HCO3-+H+=H2O+CO2����
��4���ҡ������������е��ӻ����ͷֱ���sp2��sp3��
��5������ͭ����Ķѻ���ʽ��ͼ��ʾ���辧���߳�Ϊa cm������٤��������NA��ʾ������ԭ�ӵ���λ��Ϊ12��������ܶ�Ϊ$\frac{256}{{a}^{3}{N}_{A}}$g•cm-3��Ҫ��д����ʽ�����Բ�����
 ����������Ӧ����㷺�Ľ��ȡ���ʹ�Ϳ���ҩ������ˮ���������ֽ⣬�ֽ��¶�Ϊ128�桫135�森ijѧϰС����ʵ������ˮ���ᣨ���ǻ������ᣩ�������[��CH3CO��2O]Ϊ��Ҫԭ�Ϻϳɰ�˾ƥ�֣���Ӧԭ�����£�
����������Ӧ����㷺�Ľ��ȡ���ʹ�Ϳ���ҩ������ˮ���������ֽ⣬�ֽ��¶�Ϊ128�桫135�森ijѧϰС����ʵ������ˮ���ᣨ���ǻ������ᣩ�������[��CH3CO��2O]Ϊ��Ҫԭ�Ϻϳɰ�˾ƥ�֣���Ӧԭ�����£�
�Ʊ����������������£�
������+ˮ����$\stackrel{Ũ����}{��}$$\stackrel{ҡ��}{��}$$\stackrel{85-90������}{��}$$\stackrel{��ȴ}{��}$$��_{ϴ��}^{��ѹ����}$�ֲ�Ʒ
��Ҫ�Լ��Ͳ�Ʒ�������������±���ʾ��
| ���� | ��Է������� | �۵��е㣨�棩 | ˮ |
| ˮ���� | 138 | 158���۵㣩 | �� |
| ������ | 102 | 139.4���е㣩 | ��ˮ�� |
| ����ˮ���� | 180 | 135���۵㣩 | �� |
��1���Ʊ���˾ƥ��ʱ��Ҫʹ�ø����������ԭ������������ˮ��Ӧ��
��2���ϳɰ�˾ƥ��ʱ������ʵļ��ȷ�����ˮԡ���ȣ�
��3���ᴿ�ֲ�Ʒ�������£����Ȼ���װ����ͼ��
�ֲ�Ʒ$��_{��ʯ}^{��������}$$��_{����}^{����}$$\stackrel{���ȹ���}{��}$$��_{��ѹ����}^{��ȴ}$$��_{����}^{ϴ��}$����ˮ����

��ʹ���¶ȼƵ�Ŀ���ǿ��Ƽ��ȵ��¶ȣ���ֹ����ˮ���������ֽ⣮
������ˮ������������a���a����b������
�۳��ȹ��˵�ԭ���Ƿ�ֹ����ˮ����ᾧ������
������˵����ȷ����abc����ѡ����ĸ����
a�������ᴿ�������������������������ܼ�
b�������ᴿ�ֲ�Ʒ�ķ������ؽᾧ
c�����������ᴿ���̿��Եó���˾ƥ�������������е��ܽ�ȵ���ʱС
d����������ɫʯ����Һ�жϲ�Ʒ���Ƿ���δ��Ӧ���ˮ����
��4����ʵ����ԭ��������2.0gˮ���ᡢ5.0mL����������=1.08g/cm3�������ճƵò�Ʒ����Ϊ2.2g������������ˮ����IJ���Ϊ84.3%���ðٷ�����ʾ��С�����һλ����
 ̫���ܵĿ�������������Դ�о���ռ����Ҫ��λ��������̫���ܵ��Ƭ�ڼӹ�ʱ��һ���������ͭ��ﴡ����ء����ȣ��ش��������⣺
̫���ܵĿ�������������Դ�о���ռ����Ҫ��λ��������̫���ܵ��Ƭ�ڼӹ�ʱ��һ���������ͭ��ﴡ����ء����ȣ��ش��������⣺
 $\stackrel{+HCN}{��}$
$\stackrel{+HCN}{��}$
 ��
�� ��
��


 �����ĺϳ�·�ߣ��Լ����ܼ���ѡ���ϳ�·�߲��ա���֪���е���д��ʽ��
�����ĺϳ�·�ߣ��Լ����ܼ���ѡ���ϳ�·�߲��ա���֪���е���д��ʽ�� ��
��