��Ŀ����
8������X��ij���;�ˮ�����м��壬�����Կ������Ȼ�������180����������һ����A�����ʵ���֮��1��2��ɣ����ܱ������м���8.75g Xʹ֮��ȫ�ֽ⣬��ȴ��ɵõ�3.2g����������B��0.448L��ɫ����D�����������Ϊ��״������4.27g��Ͼ���E��B����ϡ����μ�KSCN��Һ�����Һ��Ѫ��ɫ��D������ʹƷ����ɫ����ش��������⣺
��1��X�Ļ�ѧʽΪAlCl3•2FeSO4��
��2����A�����������������գ�ʹ��ֽ⣬���ɵ����ʵ�����B��D����һ�ֻ������A�ֽ�Ļ�ѧ����ʽΪ2FeSO4$\frac{\underline{\;����\;}}{\;}$SO2��+SO3+Fe2O3��
��3����E��Ͼ�������ˮ�����Һ����μ������ϡNaOH��Һ���ù��̵��ܷ�Ӧ�����ӷ���ʽΪAl3++2H++6OH-=AlO2-+4H2O��
E��Ͼ�����ij��������һ���������ܺ�KI���巴Ӧ��д���÷���ʽSO3+2KI=I2+K2SO3��
��4�������£������ܱ������г�ʱ������X�������л�������һ�����壬�����ʽ��O2�������ʵ�鷽����֤֮������ͨ������NaOH��Һ�У��ռ���������һ�������ǵı����������У�����ȼ����˵����O2��
���� ����������B����ϡ����μ�KSCN��Һ�����Һ��Ѫ��ɫ��˵��B�к���+3��Fe����BΪFe2O3����ɫD������ʹƷ����ɫ����DΪSO2����Ԫ���غ��֪A�к���Fe��S��OԪ�أ�A���ȷֽ��������������Ͷ�����������AΪFeSO4��X�����ΪAlCl3•2FeSO4�������������ʵ���Ϊ$\frac{3.2g}{160g/mol}$=0.02mol�����ɶ�������Ϊ$\frac{0.448L}{22.4L/mol}$=0.02mol����Fe��Sԭ��Ϊ1��1��֪����SO3Ϊ0.02mol��4.27g��Ͼ���EΪAlCl3��SO3��AlCl3�����ʵ���Ϊ$\frac{4.27g-0.02mol��80g/mol}{133.5g/mol}$=0.02mol��
��� �⣺����������B����ϡ����μ�KSCN��Һ�����Һ��Ѫ��ɫ��˵��B�к���+3��Fe����BΪFe2O3����ɫD������ʹƷ����ɫ����DΪSO2����Ԫ���غ��֪A�к���Fe��S��OԪ�أ�A���ȷֽ��������������Ͷ�����������AΪFeSO4��X�����ΪAlCl3•2FeSO4�������������ʵ���Ϊ$\frac{3.2g}{160g/mol}$=0.02mol�����ɶ�������Ϊ$\frac{0.448L}{22.4L/mol}$=0.02mol����Fe��Sԭ��Ϊ1��1��֪����SO3Ϊ0.02mol��4.27g��Ͼ���EΪAlCl3��SO3��AlCl3�����ʵ���Ϊ$\frac{4.27g-0.02mol��80g/mol}{133.5g/mol}$=0.02mol��
��1��X�Ļ�ѧʽΪAlCl3•2FeSO4���ʴ�Ϊ��AlCl3•2FeSO4��
��2��A�ֽ�Ļ�ѧ����ʽΪ��2FeSO4$\frac{\underline{\;����\;}}{\;}$SO2��+SO3+Fe2O3��
�ʴ�Ϊ��2FeSO4$\frac{\underline{\;����\;}}{\;}$SO2��+SO3+Fe2O3��
��3����E��Ͼ�������ˮ�����Һ����������Ӧ�������ᣬ���������Ȼ��������ʵ�����ȣ���μ������ϡNaOH��Һ���ù��̵��ܷ�Ӧ�����ӷ���ʽΪ��Al3++2H++6OH-=AlO2-+4H2O��
E�����е�SO3��һ�������ܺ�KI���巴Ӧ���÷�Ӧ����ʽΪSO3+2KI=I2+K2SO3��
�ʴ�Ϊ��Al3++2H++6OH-=AlO2-+4H2O��SO3+2KI=I2+K2SO3��
��4�����ڸ����³�ʱ������X�����ɵ����������ٷֽ����ɶ����������������һ���������ʽ��O2�����������ķ���Ϊ��������ͨ������NaOH��Һ�У��ռ���������һ�������ǵı����������У�����ȼ����˵����O2��
�ʴ�Ϊ��O2��������ͨ������NaOH��Һ�У��ռ���������һ�������ǵı����������У�����ȼ����˵����O2��
���� ���⿼��������ƶϣ���Ŀ�Ƚ��ۺϣ���Ҫѧ����������Ԫ�ػ�����֪ʶ��ȷ��A������ǽ���Ĺؼ�����Ŀ�ѶȽϴ�

ע��
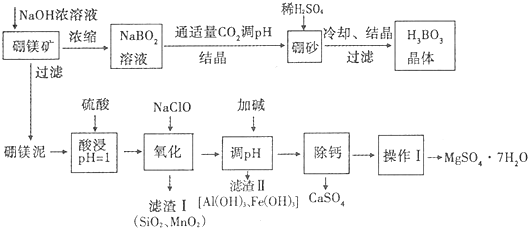
����þ�����Ҫ�ɷ���MgO��ռ40%��������CaO��MnO��Fe2O3��FeO��Al2O3��SiO2�����ʣ�
���������ƽ�ⳣ��K=5.8��10-10
��
| ������ | Mg��OH��2 | Fe��OH��2 | Fe��OH��3 | Al��OH��3 |
| Ksp����ֵ | 10-11 | 10-16 | 10-38 | 10-33 |
| �¶ȣ��棩 | 40 | 50 | 60 | 70 |
| MgSO4 | 30.9 | 33.4 | 35.6 | 36.9 |
| CaSO4 | 0.210 | 0.207 | 0.201 | 0.193 |
��2����������ӿɱ�ʾΪB��OH��4-��д���������ķ���ʽH3BO3+H2O?B��OH��4-+H+
��3��N�ζ����ⶨ���ᾧ��Ĵ��ȣ��о������������0.1mol•L-1NaOH��Һֱ�ӵζ�������Һ���ζ����������ߢ���ʾ����������Һ�м����Ԫ�����ٵζ������ߢ���ʾ����������ܷ���ǿ��ֱ�ӵζ�������Һ������ ����ܡ����ܡ����������ǣ�û�����Ե�pHͻ���������ж��յ㣬����ɽϴ�����
��4������þ����������Һ�У������NaClO����Mn2+��Ӧ����MnO2�������÷�Ӧ�����ӷ���ʽ��ClO-+Mn2++H2O=MnO2��+Cl-+2H+��
��5���Ӽ������pHΪ4.7ʱ���������ӱ����ȫ������������Ũ��С�ڻ����1��l0-5mol•L-1ʱ��������Ϊ�����ӳ�����ȫ��
��6�������ơ��ǽ�MgSO4��CaSO4�����Һ�е�CaSO4��ȥ�������ϱ����ݣ���Ҫ˵�����ƵIJ�����������Ũ���ᾧ�����ȹ��ˣ�
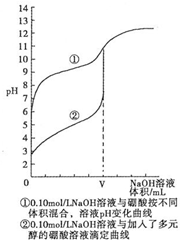
 ����Cu2O���ھ��������Ĵ����ܶ��ܵ���ע���±�Ϊ��ȡCu2O�����ַ�����
����Cu2O���ھ��������Ĵ����ܶ��ܵ���ע���±�Ϊ��ȡCu2O�����ַ�����| ����I | ��̼���ڸ��������»�ԭCuO |
| ����II | ���£�N2H4����ԭ����Cu��OH��2 |
| ����III | ��ⷨ����ӦΪ2Cu+H2O$\frac{\underline{\;���\;}}{\;}$Cu2O+H2�� |
C��s��+$\frac{1}{2}$O2��g��=CO��g����H=-bkJ•mol-1
Cu��s��+$\frac{1}{2}$O2��g��=CuO��s����H=-ckJ•mol-1
��I�����ķ�Ӧ��2Cu O��s��+C��s��=Cu2O��s��+CO��g������H=2c-a-bkJ•mol-1��
��2����ҵ�Ϻ����÷���I��ȡCu2O�������ڷ���I��Ӧ���������ƣ������²������ή��Cu2O���ʣ������ԭ�����¶Ȳ�����������Cu��
��3������IIΪ������������Һ̬�£�N2H4����ԭ����Cu��OH��2���Ʊ�����Cu2O��ͬʱ�ų�N2��
���Ʒ��Ļ�ѧ����ʽΪ4Cu��OH��2+N2H4$\frac{\underline{\;����\;}}{\;}$2Cu2O+6H2O+N2��
��4������III�������ӽ���Ĥ���Ƶ��Һ��OH-��Ũ�ȶ��Ʊ�����Cu2O��װ����ͼ��ʾ��д���缫��Ӧʽ
��˵����װ���Ʊ�Cu2O��ԭ�������缫��Ӧ��2H++2e-=H2����c��OH-������ͨ�������ӽ���Ĥ���������ң������缫��Ӧ��2 Cu-2e-+2OH-=Cu2O+H2O�����Cu2O��
��5������ͬ���ܱ������У����������ַ����Ƶõ�Cu2O�ֱ���д��ֽ�ˮ��ʵ�飺
2H2O��g��$?_{Cu_{2}O}^{����}$2H2��g��+O2��g����H��0��ˮ������Ũ�ȣ�mol/L����ʱ��t��min��
�仯���±���ʾ��
| ��� | Cu2O a�� | �¶� | 0 | 10 | 20 | 30 | 40 | 50 |
| �� | ����II | T1 | 0.050 | 0.0492 | 0.0486 | 0.0482 | 0.0480 | 0.0480 |
| �� | ����III | T1 | 0.050 | 0.0488 | 0.0484 | 0.0480 | 0.0480 | 0.0480 |
| �� | ����III | T2 | 0.10 | 0.094 | 0.090 | 0.090 | 0.090 | 0.090 |
a��ʵ����¶ȣ�T2��T1
b��ʵ���ǰ20min��ƽ����Ӧ����v��O2��=7��10-5mol•L-1•min-1
c��ʵ��ڱ�ʵ������õ�Cu2O��Ч�ʸ�
d�� ʵ��١��ڡ��۵Ļ�ѧƽ�ⳣ���Ĺ�ϵ��K1=K2��K3��
| A�� | �������ͳ�Ʒ����������о���ʵ�鷽����ģ�ͻ��������ǻ�ѧ�о��ij��÷��� | |
| B�� | ����������������ֹ����ȡ��������ͭ��ú��������ȡ���ȹ��̶��漰��ѧ�仯 | |
| C�� | ��2016��1��1�ſ�ʼ�㽭ʡ���ͱ��ɡ���������ߵ�����V�������Ⲣ����ζ�����������ŷŵ������� | |
| D�� | ��������������Ƽ����ķ�չ����ʹ���Ӿ���ܡ�����оƬ�����ӵ��ߡ����Ӽ�����Ȼ�ѧ�����õ��㷺��Ӧ�� |
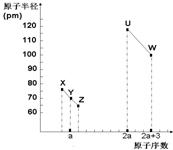 X��Y��Z��U��W���ֶ����ڷǽ���Ԫ�أ����ǵ�ԭ�Ӱ뾶��ԭ����������ͼ��ϵ��������XZ��ˮú������Ҫ�ɷ�֮һ������˵������ȷ���ǣ�������
X��Y��Z��U��W���ֶ����ڷǽ���Ԫ�أ����ǵ�ԭ�Ӱ뾶��ԭ����������ͼ��ϵ��������XZ��ˮú������Ҫ�ɷ�֮һ������˵������ȷ���ǣ�������| A�� | U��X��W ����Ԫ������������ˮ��������������ǿ | |
| B�� | ��Y��Z��������Ԫ���γɵĻ�������ֻ�й��ۼ� | |
| C�� | XZ2��YZ2��X60�Ļ�ѧ�����ͺ;������Ͷ���ͬ | |
| D�� | TԪ����Uͬ����������һ���ڣ����γɻ�����TW4��TZ2��T3Y4 |
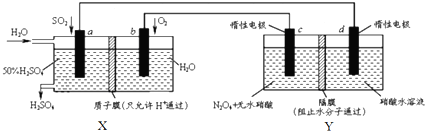
| A�� | X��ԭ��أ��ܹ��������ᣮY�ǵ��أ��ܹ�����N2O5 | |
| B�� | c�缫�ĵ缫��Ӧ����ʽΪ��N2O4+2HNO3-2e-=2N2O5+2H+ | |
| C�� | ����·��ͨ��2mol e-��X��Y�и���1molH+�����Ǩ�Ƶ��ұ� | |
| D�� | Ϊ��֤X������������������䣬������n��so2����n��H2O��=1��7.4 |
| A�� | �ǽ����ԣ�Y��X��W | |
| B�� | ����̬�⻯������ȶ��ԣ�Y��X | |
| C�� | ������ZW��XY�л�ѧ��������ͬ | |
| D�� | X��W������������ˮ�����Ϊǿ�� |
| ʵ���� | �� | �� | �� | �� | �� |
| c��I-��/mol/L | 0.040 | 0.080 | 0.080 | 0.160 | 0.120 |
| c��S2O${\;}_{8}^{2-}$��/mol/L | 0.040 | 0.040 | 0.080 | 0.020 | 0.040 |
| t/s | 88.0 | 44.0 | 22.0 | 44.0 | t1 |
��1����ʵ���Ŀ���ǣ��о���Ӧ��I-��S2O82-��Ũ�ȶԷ�Ӧ���ʵ�Ӱ�죮
��2�����ݢ١��ڡ�������ʵ������ݣ��Ʋ���ɫʱ��t1=29.3s��
��3���¶ȶԸ÷�Ӧ�ķ�Ӧ���ʵ�Ӱ�����һ����ɣ�����40���½��б�Ţ۶�ӦŨ�ȵ�ʵ�飬��ɫʱ��t2�ķ�ΧΪA������ĸ����
A����22.0s B��22.0��44.0s C����44.0s D�����ݲ��㣬���ж�
��4��ͨ�������Ƚ��ϱ����ݣ��õ��Ľ��۷�Ӧ�����뷴Ӧ����ʼŨ�ȳ˻������ȣ�����ɫʱ���뷴Ӧ����ʼŨ�ȳ˻��ɷ��ȣ�
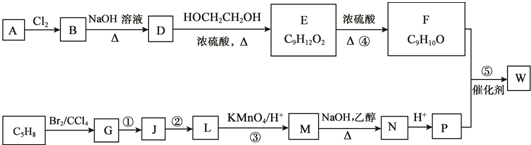
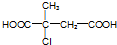 ��
�� ��D�к��������ŵ��������ǻ����ܵķ�Ӧ��������ȥ��Ӧ��
��D�к��������ŵ��������ǻ����ܵķ�Ӧ��������ȥ��Ӧ��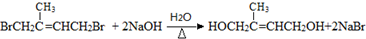 ��
�� ��
��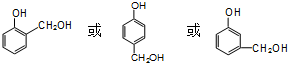 ��
��