��Ŀ����
18�� ���ʽṹ�����������ʣ��ش��������⣺
���ʽṹ�����������ʣ��ش��������⣺A��B��C��DΪԭ�������������������Ԫ�أ�A2-��B+������ͬ�ĵ��ӹ��ͣ�C��DΪͬ����Ԫ�أ�C�������������������������3����DԪ���������һ��δ�ɶԵ��ӣ��ش��������⣺
��1������Ԫ���е縺��������O����Ԫ�ط��ţ�������Cԭ�ӵĺ�������Ų�ʽΪ1s22s22p63s23p3��
��2������A������ͬ�������壬���зе�ߵ���O3�������ʽ����B���⻯�������ľ�������Ϊ���Ӿ��壮
��3��������D2A�����幹��ΪV�Σ�����ԭ�ӵ��ӻ��������Ϊsp3��
��4��A��B�ܹ��γɻ�����F���侧���ṹ��ͼ��ʾ�����ɫ��ΪA��С��ɫ��ΪB������������a=0.566nm��F�Ļ�ѧʽΪNa2O����ʽ���㾧��F���ܶȣ�g��cm-3��2.27g•cm-3��
���� C�������������������������3����ӦΪPԪ�أ�C��DΪͬ����Ԫ�أ���ӦΪ��������Ԫ�أ�DԪ���������һ��δ�ɶԵ��ӣ�ӦΪClԪ�أ�A2-��B+������ͬ�ĵ��ӹ��ͣ����ԭ��������ϵ��֪AΪOԪ�أ�BΪNaԪ�أ�
��1��ͬ����������ҵ縺������ClԪ������������Ԫ�ر��ָ��ۣ�����Ԫ�ص縺������ΪOԪ�أ�CΪPԪ�أ�ԭ�Ӻ��������Ϊ15�������������ԭ��ԭ����д��������Ų�ʽ��
��2��AΪOԪ�أ���O2��O3����ͬ�������壬�����ڷ��Ӿ��壬���ԭ������Խ�е�Խ�ߣ�NaH�������Ӿ��壻
��3��������D2AΪCl2O������Oԭ�ӹµ��Ӷ������۲���Ӷ���=�ļ���Ŀ+�µ��Ӷ���������ȷ���ռ乹�ͣ�
��4�����ݾ�̯�����㾧����Na��Oԭ����Ŀ��ȷ����ѧʽ������������������ɼ����ܶȣ�
��� �⣺C�������������������������3����ӦΪPԪ�أ�C��DΪͬ����Ԫ�أ���ӦΪ��������Ԫ�أ�DԪ���������һ��δ�ɶԵ��ӣ�ӦΪClԪ�أ�A2-��B+������ͬ�ĵ��ӹ��ͣ����ԭ��������ϵ��֪AΪOԪ�أ�BΪNaԪ�أ�
��1��ͬ����������ҵ縺������ClԪ������������Ԫ�ر��ָ��ۣ�����Ԫ�ص縺������ΪOԪ�أ�CΪPԪ�أ�ԭ�Ӻ��������Ϊ15�������������ԭ��ԭ������������Ų�ʽΪ��1s22s22p63s23p3��
�ʴ�Ϊ��O��1s22s22p63s23p3��
��2��AΪOԪ�أ���O2��O3����ͬ�������壬���߶�Ӧ�ľ��嶼Ϊ���Ӿ��壬��O3���ԭ�������ϴ��»����ϴе�ϸߣ�B���⻯��ΪNaH���������Ӿ��壬
�ʴ�Ϊ��O3�����Ӿ���
��3��������D2AΪCl2O��OΪ����ԭ�ӣ��γ�2���Ҽ����µ��Ӷ���Ϊ$\frac{6-1��2}{2}$=2��������ԭ�ӵļ۲���Ӷ���Ϊ4�����幹��ΪV�Σ�Oԭ��Ϊsp3�ӻ���
�ʴ�Ϊ�������Σ�sp3��
��4��A��B�ܹ��γɻ�����FΪ���ӻ����������λ�ھ����Ķ�������ģ�������λ�ھ��������ģ���Na�ĸ���Ϊ8��O�ĸ���Ϊ8��$\frac{1}{8}$+6��$\frac{1}{2}$=4��N��Na����N��O��=2��1�����γɵĻ�����ΪNa2O��
����������Ϊ$\frac{4��62g/mol}{6.02��1{0}^{23}mo{l}^{-1}}$�����������Ϊ��0.566��10-7��cm3������F���ܶ�Ϊ$\frac{4��62g/mol}{6.02��1{0}^{23}mo{l}^{-1}}$���£�0.566��10-7��cm3=2.27g•cm-3��
�ʴ�Ϊ��Na2O��2.27g•cm-3��
���� �����Ƕ����ʽṹ�����ʵĿ��飬�漰��������Ų����е�Ƚϡ��ӻ���ʽ��ռ乹���жϡ���������ȣ����ؿ���ѧ���ķ�������������ע����Ԫ������Ԫ�ص縺�ԱȽϣ��Ѷ��еȣ�

| A�� | 0.2mol | B�� | 0.25 mol | C�� | 0.35 mol | D�� | 0.45 mol |
 һ�ֹ⻯ѧ��صĽṹ��ͼ��ʾ������ܷ�ӦΪAgCl��s��+Cu+��aq���TAg��s��+Cu2+��aq��+Cl-��aq�������й��ڸõ���ڹ���ʱ��˵������ȷ���ǣ�������
һ�ֹ⻯ѧ��صĽṹ��ͼ��ʾ������ܷ�ӦΪAgCl��s��+Cu+��aq���TAg��s��+Cu2+��aq��+Cl-��aq�������й��ڸõ���ڹ���ʱ��˵������ȷ���ǣ�������| A�� | ����108 g����ת�Ƶ��Ӹ���Ϊ1 mol | |
| B�� | Cu+�ڸ�������������Ӧ | |
| C�� | Ag�缫���ã�Agʧ���ӷ���������Ӧ | |
| D�� | Cl-�ɸ���Ǩ�Ƶ����� |

| A�� | ���ЦҼ����Цм� | |
| B�� | O-H���ļ���ǿ��C-H���ļ��� | |
| C�� | �Ǽ��Է��� | |
| D�� | �����ʵķ���֮�䲻���γ����������������ˮ�����γ���� |
| A�� | c��PO43-����Na3PO4��Na2HPO4��NaH2PO4��H3PO4 | |
| B�� | c��CO32-������NH4��2CO3��Na2CO3��NaHCO3��NH4HCO3 | |
| C�� | c��NH4+������NH4��2SO4����NH4��2CO3��NH4HSO4��NH4Cl | |
| D�� | c��S2-����Na2S��NaHS��H2S |
| A�� | ���ᣨCH3COOH�� | B�� | �������ᣨH2N-CH2-COOH�� | ||
| C�� | ���أ� �� �� | D�� | �������ͣ� �� �� |
| A�� | ����ͨ������У�CH3COOH+NH3�TCH3COO-+NH4+ | |
| B�� | ��̼����þ��Һ�мӹ���ʯ��ˮ��Mg2++2HCO3-+Ca2++2OH-�TCaCO3��+2H2O+MgCO3�� | |
| C�� | ����ʯ��ˮ��ϡ���ᷴӦ��Ca��OH��2+2H+�TCa2++2H2O | |
| D�� | ϡ�������ͭƬ�ϣ�Cu+2H+�TCu2++H2�� |



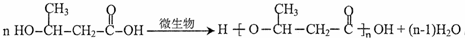 ��
�� ��һ�������л����������ϣ����ڴ��������£���������Ӧ�Ļ�ѧ����ʽ��
��һ�������л����������ϣ����ڴ��������£���������Ӧ�Ļ�ѧ����ʽ��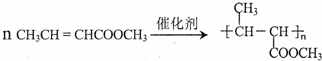 ��
��