��Ŀ����
2��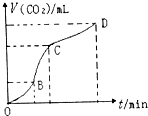 ��ͼ���ô�����̼�����ϡ���ᷴӦ��ȡ������̼���������Ƶ�ͼ���������ͼ���жϣ������й�˵��������ǣ�������
��ͼ���ô�����̼�����ϡ���ᷴӦ��ȡ������̼���������Ƶ�ͼ���������ͼ���жϣ������й�˵��������ǣ�������| A�� | CD�ζ�����̼��������С��BC�ζ�����̼�������� | |
| B�� | ��OB��BC��CD�����У�BC�εķ�Ӧ������죬ԭ���Ǹ÷�Ӧ���ȣ��¶���BC������Ҫ���� | |
| C�� | ��ͬ�����£���������Ӧ����Һ�м���NaCl��Һ�ɽ��ͻ�ѧ��Ӧ������ | |
| D�� | OB�η�Ӧ��Ũ�ȴ�����OB�η�Ӧ������� |
���� A�������������ʾ����������̼��������ش�
B������Ũ�ȡ��¶ȶԻ�ѧ��Ӧ���ʵ�Ӱ������֪ʶ���ش�
C��̼��ƺ�����ķ�Ӧʵ����̼��ƺ�������֮��ķ�Ӧ����������Ũ��֮����ϵ����Һ�м���NaCl��Һ�൱�ڼ�ˮϡ�ͣ�
D����λʱ���ڲ����������������Ӧ������죬����ͼʾ���ݻش�
��� �⣺A������ͼʾ�������ʾ����������̼�����֪����CD�ζ�����̼��������С��BC�ζ�����̼������������A��ȷ��
B������ͼʾ�������ʾ����������̼�����֪������OB��BC��CD�����У�BC�εķ�Ӧ������죬ԭ���Ǹ÷�Ӧ���ȣ��¶��������ʼӿ죬�¶���BC������Ҫ���ã���B��ȷ��
C��������Ӧ����Һ�м���NaCl��Һ���൱�ڽ�����ϡ�ͣ����Կɽ��ͻ�ѧ��Ӧ�����ʣ���C��ȷ��
D����λʱ���ڲ����������������Ӧ������죬����ͼʾ�������ʾ����������̼�����֪������OB��BC��CD�����У�BC�εķ�Ӧ������죬��D����
��ѡD��
���� ���⿼��Ӱ�컯ѧ��Ӧ���ʵ����أ�ע�ⷴӦ����Һ�м���NaCl��Һ���൱�ڽ�����ϡ�ͣ����ͷ�Ӧ����Ϊ���Ĺؼ�����ȷ����Ӱ�����ؼ��ɽ����Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
13���������ʽΪC4H8ClBr���л��ﹲ�У�������
| A�� | 11�� | B�� | 12�� | C�� | 13�� | D�� | 14�� |
10����ͬ�����£��л���M�������ܶ�ΪH2��44������M�����������¿�ˮ��õ�����һԪ��ͱ���һԪ�����������������칹�����л���M�Ŀ��ܽṹ�У�������
| A�� | 2�� | B�� | 3�� | C�� | 4�� | D�� | 5�� |
17������˵����ȷ���ǣ�������
| A�� | 12C��C60����ͬһ��Ԫ�أ���Ϊͬ�������� | |
| B�� | 16O��18O�Dz�ͬ�ĺ��أ���Ϊͬλ�� | |
| C�� | 37Cl��35Cl����ͨ����ѧ�仯ʵ���ת�� | |
| D�� | H2O��H2O2��ͬ��Ԫ����ɣ�����Ϊͬ�������� |
7��һ�������£��ܱ�����������Ӧ2SO2��g��+O2��g��?2SO3��g��������2molSO3��g��ʱ���ų�QkJ����������˵����ȷ���ǣ�������
| A�� | ��ͬ�����£�2molSO2��g����1molO2��g�������е�����С��2molSO3��g�������е����� | |
| B�� | ��v��������v���棩ʱ�����ŷ�Ӧ���У���Ӧ������ʵ������� | |
| C�� | ��ͬ�����£�����18O2��SO2��g�����ܱ������з�Ӧ����ƽ��ʱ��18O�����ڶ����������������������� | |
| D�� | ��ͬ�����£����ܱ������м���2molSO2��g����1molO2��g����ַ�Ӧ�ų�������ΪQkJ |
11�� ���ӽ���Ĥ�ڵ��غ�ԭ��������Ŷ��ص����ã��������ӽ���Ĥ������ͨ����ͼ��ʾװ�õ��Na2SO3��Һ��ȡNaOH��Һ��H2SO4��Һ������A��B�缫Ϊ���Ե缫��������˵����ȷ���ǣ�������
���ӽ���Ĥ�ڵ��غ�ԭ��������Ŷ��ص����ã��������ӽ���Ĥ������ͨ����ͼ��ʾװ�õ��Na2SO3��Һ��ȡNaOH��Һ��H2SO4��Һ������A��B�缫Ϊ���Ե缫��������˵����ȷ���ǣ�������
 ���ӽ���Ĥ�ڵ��غ�ԭ��������Ŷ��ص����ã��������ӽ���Ĥ������ͨ����ͼ��ʾװ�õ��Na2SO3��Һ��ȡNaOH��Һ��H2SO4��Һ������A��B�缫Ϊ���Ե缫��������˵����ȷ���ǣ�������
���ӽ���Ĥ�ڵ��غ�ԭ��������Ŷ��ص����ã��������ӽ���Ĥ������ͨ����ͼ��ʾװ�õ��Na2SO3��Һ��ȡNaOH��Һ��H2SO4��Һ������A��B�缫Ϊ���Ե缫��������˵����ȷ���ǣ�������| A�� | aĤΪ�����ӽ���Ĥ | |
| B�� | ��Һ��SO${\;}_{3}^{2-}$��M�Ҿ�bĤ����D�� | |
| C�� | A���ĵ缫��ӦʽΪSO${\;}_{3}^{2-}$+2e-+H2O�TSO${\;}_{4}^{2-}$+2H+ | |
| D�� | C����Һ�����ԣ�M����Һ�����ԣ�D����Һ�ʼ��� |
7��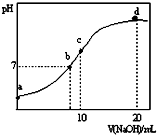 �����£���20.00mL 0.1000mol•L-1 ��NH4��2SO4��Һ����μ���0.2000mol•L-1NaOHʱ����Һ��pH������NaOH��Һ����Ĺ�ϵ��ͼ��ʾ�������ǻӷ���������˵������ȷ���ǣ�������
�����£���20.00mL 0.1000mol•L-1 ��NH4��2SO4��Һ����μ���0.2000mol•L-1NaOHʱ����Һ��pH������NaOH��Һ����Ĺ�ϵ��ͼ��ʾ�������ǻӷ���������˵������ȷ���ǣ�������
 �����£���20.00mL 0.1000mol•L-1 ��NH4��2SO4��Һ����μ���0.2000mol•L-1NaOHʱ����Һ��pH������NaOH��Һ����Ĺ�ϵ��ͼ��ʾ�������ǻӷ���������˵������ȷ���ǣ�������
�����£���20.00mL 0.1000mol•L-1 ��NH4��2SO4��Һ����μ���0.2000mol•L-1NaOHʱ����Һ��pH������NaOH��Һ����Ĺ�ϵ��ͼ��ʾ�������ǻӷ���������˵������ȷ���ǣ�������| A�� | ��a��ʾ��Һ�У�c��NH4+����c��SO42-����c��H+����c��OH-�� | |
| B�� | ��b��ʾ��Һ�У�c��NH4+��=c��Na+����c��H+��=c��OH-�� | |
| C�� | ��c��ʾ��Һ�У�c��SO42-��+c��H+��=c��NH3•H2O ��+c��OH-�� | |
| D�� | ��d��ʾ��Һ�У�c��NH3•H2O ����c��SO42-����c��OH-����c��NH4+�� |