��Ŀ����
7��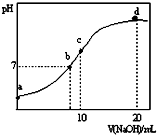 �����£���20.00mL 0.1000mol•L-1 ��NH4��2SO4��Һ����μ���0.2000mol•L-1NaOHʱ����Һ��pH������NaOH��Һ����Ĺ�ϵ��ͼ��ʾ�������ǻӷ���������˵������ȷ���ǣ�������
�����£���20.00mL 0.1000mol•L-1 ��NH4��2SO4��Һ����μ���0.2000mol•L-1NaOHʱ����Һ��pH������NaOH��Һ����Ĺ�ϵ��ͼ��ʾ�������ǻӷ���������˵������ȷ���ǣ�������| A�� | ��a��ʾ��Һ�У�c��NH4+����c��SO42-����c��H+����c��OH-�� | |
| B�� | ��b��ʾ��Һ�У�c��NH4+��=c��Na+����c��H+��=c��OH-�� | |
| C�� | ��c��ʾ��Һ�У�c��SO42-��+c��H+��=c��NH3•H2O ��+c��OH-�� | |
| D�� | ��d��ʾ��Һ�У�c��NH3•H2O ����c��SO42-����c��OH-����c��NH4+�� |
���� A��a����Һ�У�笠�����ˮ�����Һ�����ԣ���笠�����ˮ�������
B��b����Һ�У���Һ�����ԣ���c��H+��=c��OH-�����������ʵ������жϣ�
C��c����Һ�д��ڵ���غ�������غ㣬���ݵ���غ㡢�����غ��жϣ�
D��d����Һ�У�����ǡ�÷�Ӧ���������ơ�һˮ�ϰ������������ǰ�ˮŨ�ȵ�һ�룮
��� �⣺A��a����Һ�У�笠�����ˮ�����Һ�����ԣ���c��H+����c��OH-����笠�����ˮ��̶Ƚ�С����������غ�֪c��NH4+����c��SO42-����������Һ������Ũ�ȴ�С˳����c��NH4+����c��SO42-����c��H+����c��OH-������A��ȷ��
B��b����Һ�У���Һ�����ԣ���c��H+��=c��OH-�������ݵ���غ��c��H+��+c��NH4+��+c��Na+��=2c��SO42-��+c��OH-��������淋����Խ�����Ҫʹ�����Һ�����ԣ�����������������Ƽ��ɣ�����c��NH4+����c��Na+������B����
C��c����Һ�У���Һ�ʼ��ԣ�����c��H+����c��OH-������Һ�е������ǵ����ʵ���Ũ�ȵ�����李������ơ�һˮ�ϰ������ݵ���غ��c��H+��+c��NH4+��+c��Na+��=2c��SO42-��+c��OH-�������������غ�ã����������غ��c��NH4+��+c��NH3•H2O��=2c��SO42-��=2c��Na+�������Ե�c��SO42-��+c��H+��=c��NH3•H2O ��+c��OH-������C��ȷ��
D��d����Һ�У�����ǡ�÷�Ӧ���������ơ�һˮ�ϰ������������ǰ�ˮŨ�ȵ�һ�룬һˮ�ϰ�����̶Ƚ�С������c��NH3•H2O ����c��SO42-������D��ȷ��
��ѡB��
���� ���⿼��������Ũ�ȴ�С�ıȽϣ���ȷͼ�������߱仯���ơ�������ÿһ��������ʼ��������ǽⱾ��ؼ����ٽ�ϵ���غ㡢�����غ������������Ŀ�Ѷ��еȣ�

 ��У����ϵ�д�
��У����ϵ�д�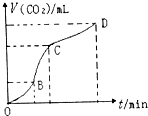 ��ͼ���ô�����̼�����ϡ���ᷴӦ��ȡ������̼���������Ƶ�ͼ���������ͼ���жϣ������й�˵��������ǣ�������
��ͼ���ô�����̼�����ϡ���ᷴӦ��ȡ������̼���������Ƶ�ͼ���������ͼ���жϣ������й�˵��������ǣ�������| A�� | CD�ζ�����̼��������С��BC�ζ�����̼�������� | |
| B�� | ��OB��BC��CD�����У�BC�εķ�Ӧ������죬ԭ���Ǹ÷�Ӧ���ȣ��¶���BC������Ҫ���� | |
| C�� | ��ͬ�����£���������Ӧ����Һ�м���NaCl��Һ�ɽ��ͻ�ѧ��Ӧ������ | |
| D�� | OB�η�Ӧ��Ũ�ȴ�����OB�η�Ӧ������� |
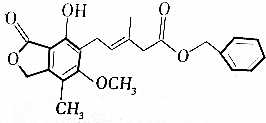
| A�� | �����ʵĻ�ѧʽ��C23H24O6 | |
| B�� | 1mol�û�������������9molH2�ӳ� | |
| C�� | ����KMnO4��Һ����ˮ��������ʷ���������ԭ��Ӧ����ɫ | |
| D�� | ��������FeCl3��Һ���ÿ��Ժ�ɫ���÷�Ӧ�����ڼ������еķ��ǻ� |
| A�� | CH3COOH��Ũ�ȱ�NaOH��Һ��Ӧ�������������� | |
| B�� | CH3COOH��Һ��ˮ�ĵ���̶ȴ���NaOH��Һ��ˮ�ĵ���̶� | |
| C�� | ��Ӧ����Һ�е�����Ũ��һ����c��CH3COO-����c��Na+����c��H+����c��OH-�� | |
| D�� | ��Ӧ�����Һ��c��Na+����c��CH3COO-��������� |
| A�� | ���� | B�� | �� | C�� | ������� | D�� | �Ҷ�ȩ��OHC-CHO�� |
| A�� | �����������ɫ����ƿ�� | |
| B�� | �����ƴ����ʢ��ú�͵��Լ�ƿ�� | |
| C�� | ����������Һ������ĥ�ڲ��������Լ�ƿ�� | |
| D�� | Ũ���ᱣ����ĥ�ڲ�������ɫ�Լ�ƿ�У������������� |
 �Ʊ�
�Ʊ�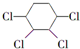
 B��
B�� ��
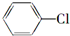
�� +2NaOH$��_{��}^{��}$
+2NaOH$��_{��}^{��}$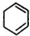 +2NaCl+2H2O
+2NaCl+2H2O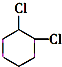 �ĺ�����Ԫ̼����ͬ���칹�壺
�ĺ�����Ԫ̼����ͬ���칹�壺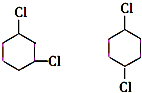
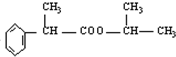
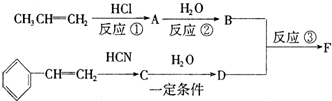
 ��
��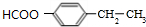 ��
��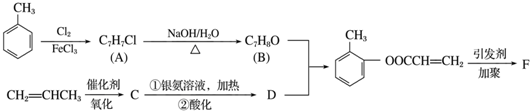
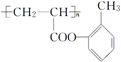 ��
�� ����ṹ��ʽ����
����ṹ��ʽ����