��Ŀ����
3����NO2��������һ������ͨ������ˮ�У��õ���Һ�����������Һ������˵����ȷ���ǣ�������| A�� | ��NO2��O2���������4��3�������Һ����������������� | |
| B�� | ��һ������CuͶ����Һ�У�ֻ����1.12L��������ĺ���ɫ���壬��Ӧ��ͭ��1.6g | |
| C�� | ����Һ��ǿ���¹��գ��������ݣ���Һ��dz�ƣ��������ݣ���������NO2 | |
| D�� | ��dz�Ƶ�������ͨ������������ʹ��pHֵ���ͣ���Һ�������� |
���� ����������ˮ��Ӧ���������һ��������3NO2+H2O=2HNO3+NO��һ���������������ɶ���������2NO+O2=2NO2��NO2��������ˮ��Ӧ��4NO2+O2+2H2O=4HNO3��NO��������ˮ��Ӧ��4NO2+3O2+2H2O=4HNO3��
A����ͬ�����£�NO2��O2�����֮�ȵ��������ʵ���֮�ȣ�NO2��O2�����ʵ���֮��Ϊ4��3������ʣ�ࣻ
B��ͭ��һ�������ᷴӦ����������Ϊ���ᣬͭ����ԭ�������ݵ�ʧ�����غ���㣻
C������������ˮ��Ӧ���������һ��������
D����dz�Ƶ�������ͨ�������������ж�������ת��Ϊ���ᣮ
��� �⣺A����ͬ�����£�NO2��O2�����֮�ȵ��������ʵ���֮�ȣ�NO2��������ˮ��Ӧ��4NO2+O2+2H2O=4HNO3������ʣ�࣬��Һ�������Ậ���ܽ���������ʣ���A����
B������Nԭ���غ㣬������������������ʵ���n��HNO3��=n��NO2��=$\frac{1.12L}{22.4L/mol}$=0.05mol��ת�Ƶ���Ϊ0.05mol����Ӧ��ͭn��Cu��=$\frac{0.05mol}{2}$=0.025mol��m��Cu��=0.025mol��64g/mol=1.6g����B��ȷ��
C������������ˮ��Ӧ���������һ��������3NO2+H2O=2HNO3+NO��������NO����C����
D��dz�Ƶ�����Ϊ���������ܽ����е���ɫ��ͨ������������Ӧ��NO2��������ˮ��Ӧ��4NO2+O2+2H2O=4HNO3��pHֵ���ͣ���Һ�������ӣ���D����
��ѡB��
���� ���⿼�鵪���仯��������ʣ����յ������������ʡ�������ԭ��Ӧԭ����Ӧ���ǽ��ؼ�����Ŀ�Ѷ��еȣ�

 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�
| A�� | N2������+3H2������?2NH3����������H��0 | B�� | C���̣�+CO2������?2CO����������H��0 | ||
| C�� | N2������ʮO2������?2N0����������H��0 | D�� | CaCO3���̣�?CaO���̣�+CO2����������H��0 |
��֪
 ��a��b��c�������㣩
��a��b��c�������㣩����˵����ȷ���ǣ�������
| A�� | H2��I2��HI�����еĻ�ѧ�����ǷǼ��Թ��ۼ� | |
| B�� | �Ͽ�2mol HI�����еĻ�ѧ����������ԼΪ��c+b+a��kJ | |
| C�� | ��ͬ�����£�1mol H2��g����1mol I2��g��������С��2molHI��g���������� | |
| D�� | ���ܱ������м���2mol H2��g����2mol I2��g������ַ�Ӧ��ų�������С��2a kJ |
| A�� | ҽ�þƾ���ָ��������Ϊ75%���Ҵ���Һ | |
| B�� | �ù��˵ķ������Է����������������� | |
| C�� | �����ǡ���������һ�������¶��ܷ���������Ӧ | |
| D�� | �ڵ�������Һ�м���Ũ�����Σ��磨NH4��2SO4��CuSO4�ȣ�����ʹ�����ʵ��ܽ�Ƚ��Ͷ�������������̳�֮Ϊ���� |
| A�� | ���ǵ�ˮ�⣨�Թܡ�������Һ�����Ƶ�Cu��OH��2����Һ�� | |
| B�� | ��NaOH����Һȷ��δ֪Ũ�ȵ�������Һ��ʯ����Һ����ʽ�ζ��ܡ���ƿ�� | |
| C�� | ֤�������д��ڵ�Ԫ�أ�©����ϡ���ᡢ�������������� | |
| D�� | �������������壨����FeCl3��Һ��NaOH��Һ����ͷ�ιܣ� |

| A�� | ������Һ��pH�� Ũ�ȼ�С Ũ�ȼ�С | |
| B�� | ������Һ��pH�� Ũ�ȼ�С Ũ�ȼ�С | |
| C�� | ����Һ��pH�ʵ������ְ��������ӵ�Ũ�ȿ�����ͬ | |
| D�� | pH�ı䣬�Ե��뷽ʽ��Ӱ�� |
| A�� | ������ά�ͺϳ��������л��߷��ӻ����� | |
| B�� | ʳƷ���������ʢ�й轺�����۵�С�����ɷ�ֹʳ���ܳ����������� | |
| C�� | Na2FeO4����ˮ������Ӧ����Fe��0H��3��O2������Ϊ����ˮ���������;����� | |
| D�� | ��ľ�Һ��̬���ʲ��ܻ��ʹ�ã�����ΪNH4++HCO3-=CO2��+H2O+NH3�� |
| A�� | ���ɵ����λ�ѧʽΪBmAn | |
| B�� | �����д�����һ��ˮ������ӣ�������ˮ�ⷽ��ʽΪ��Bm++mH2O?B��OH��m+mH+ | |
| C�� | ���ɵ���Ϊǿ�������� | |
| D�� | HnAΪ���ᣬ���һ�����뷽��ʽΪ��HmA?Hm-1A-+H+ |
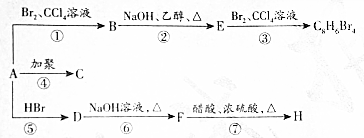
 ��D
��D ��E
��E ��
�� +2NaOH$��_{��}^{��}$
+2NaOH$��_{��}^{��}$ +NaBr��ȡ����Ӧ����
+NaBr��ȡ����Ӧ����