��Ŀ����
7�������������ɫ��������ˮ��������Ҳ�Ǹ��ܵ�صĵ缫���ϣ���ҵ��������������Ϊԭ�ϣ�ͨ�����ƣ�FeOOH���Ʊ�������أ��ɽ��������ɱ��Ҳ�Ʒ�����ţ�����������ͼ��
�ش��������⣺
��1����ͬѧ��Ϊ�������̿������ȼҵ���ϣ�д����ⱥ��ʳ��ˮ��ȡ�������ƵĻ�ѧ����ʽNaCl+H2O$\frac{\underline{\;���\;}}{\;}$NaClO+H2����
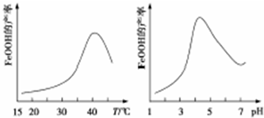
��2���Ʊ����Ƶ����ӷ���ʽΪ4Fe2++O2+6H2O=4FeOOH+8H+��ʵ���÷�Ӧ��Һ��pH���¶ȶ����Ʋ��ʵ�Ӱ����ͼ��ʾ����Ӧ�¶���ѡ��40�棻pH����4.5ʱ���Ʋ������͵���Ҫԭ����������Լ�����Fe��OH��3���࣮
��3���ø��������ˮ���������˿��Զ�ˮ�����ɱ������֮�⣬�仹ԭ���������������廹������ˮ���������ʣ���д���ø�����س�ȥˮ����CN-�����ӷ���ʽ10FeO42-+6CN-+22H2O=10Fe��OH��3�����壩+6CO32-+3N2��+14OH-��
��4����֪�������£�Ksp[Fe��OH��3]=4.0��10-38��������صľ�ˮ�������ˮ��pH�йأ�����ҺpH=2ʱ����ˮ��c��Fe3+��=0.04mol•L-1��
��5��������������У���Ԫ����������Ϊ75%������1L 2mol•L-1FeSO4��Һ���Ʊ�����Ϊ90%�ĸ������330g��
���� �Ʊ�������أ�����������+2�۵������л�ԭ�ԣ��������������ԣ����߷�Ӧ�������ƣ���ӦΪ��4Fe2++O2+6H2O=4FeOOH+8H+���������������Ʒ�Ӧ�����Ȼ��ơ��������ƺ�ˮ����Ӧ�Ļ�ѧ����ʽΪ��Cl2+2NaOH�TNaCl+NaClO+H2O�����ƺʹ������Ʒ�Ӧ���ɸ������ƣ�2FeOOH+3NaClO+4NaOH=2Na2FeO4+3NaCl+3H2O��������������Һ���ܽ�ȴ��ڸ�����أ������Ȼ��أ�2KOH+Na2FeO4 =K2FeO4+2NaOH��������������Һ��ת���ɸ�����أ����ã����˻�ôֲ�Ʒ���ݴ˷������
��� �⣺��1����ⱥ��ʳ��ˮ�������������Ϊ�������ƺ������������ķ�ӦΪ��NaCl+H2O$\frac{\underline{\;���\;}}{\;}$NaClO+H2����
�ʴ�Ϊ��NaCl+H2O$\frac{\underline{\;���\;}}{\;}$NaClO+H2����
��2������������+2�۵������л�ԭ�ԣ������������ԣ����߷���������ԭ��Ӧ�������ƣ���ӦΪ��4Fe2++O2+6H2O=4FeOOH+8H+������ͼ��40������ʱ�����Ʋ��ʽϸߣ�PH����4.5ʱ�����Լ�������������������ʹ�����Ʋ��ʽ��ͣ�
�ʴ�Ϊ��4Fe2++O2+6H2O=4FeOOH+8H+��40�棻���Լ�����Fe��OH��3���ࣻ
��3��������ؾ��������ԣ����Խ�CN-����Ϊ�����Ͷ�����̼����ȥ����������ԭΪ�����������ڼ�����Һ�ж�����̼���̼������ӣ���Ӧ�ķ���ʽΪ��10FeO42-+6CN-+22H2O=10Fe��OH��3�����壩+6CO32-+3N2��+14OH-��
�ʴ�Ϊ��10FeO42-+6CN-+22H2O=10Fe��OH��3�����壩+6CO32-+3N2��+14OH-��
��4��PH=2ʱ��C��OH-��=10-12mol/L������Ksp[Fe��OH��3]=4.0��10-38��C��Fe3+��=$\frac{4.0��1{0}^{-38}}{��1{0}^{-12}��^{3}}$mol/L=0.04mol/L��
�ʴ�Ϊ��0.04��
��5��1mol 2mol•L-1FeSO4��Һ�к���2molFeSO4��������Ԫ���غ㣬���ɸ�����ص�����Ϊ��m=2mol��75%��198g/mol��90%=330g��
�ʴ�Ϊ��330��
���� ���⿼�������ʵ��Ʊ����漰�Թ������̵����⡢������ԭ��Ӧ���������Ŀ���ѡ�������⡢�ܶȻ��ļ���ȣ����������ԭ���ǽ���Ĺؼ����Ƕ�ѧ���ۺ������Ŀ��飬��Ҫѧ���߱���ʵ�Ļ��������������������Ŀ�Ѷ��еȣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| A�� | ���ζ�ѡ��ָʾ����������Һ | |
| B�� | H�㴦����Һ��pH��3 | |
| C�� | X=7ʱ��M���Ӧ��������������Һ�������25.00 mL | |
| D�� | H��M��N������Զ�Ӧ����Һ��c��Na+��+c��H+��=c��CH3COO-��+c��OH-�� |
| A�� | ������ͭ��Ӧ�õ�NO2��N2O4��23g����ͭʧȥ�ĵ�����Ϊ0.5NA | |
| B�� | 10g 46%���Ҵ�ˮ��Һ��������ԭ����ĿΪ0.6NA | |
| C�� | ��״����8.96L��ƽ����Է�������Ϊ3.5��H2��D2����������0.3NA | |
| D�� | 1molCu��������S��ȫ��Ӧ��ת�Ƶ�����Ϊ2NA |
| A�� | ����ˮ��Ӧ | B�� | �����ڿ�����ȼ�� | ||
| C�� | ����������������Һ��Ӧ | D�� | ̼�������̼���·�Ӧ |

| A�� | ��̬�⻯����ȶ��ԣ�a��b��c | |
| B�� | ԭ�Ӱ뾶��С��a��b��c | |
| C�� | c��d��e���������Ӧ��ˮ����֮���������ܷ�Ӧ | |
| D�� | b��c�γɵĻ����������������ӵĸ�����Ϊ1��2 |

| A�� | ����ҵķ���ʽΪC13H18O2 | |
| B�� | ������뱽������ͬϵ�� | |
| C�� | 1mol ������������3mol ���������ӳɷ�Ӧ | |
| D�� | ������ڱ����Ϸ���ȡ����Ӧ����һ�ȴ�����4�� |

����˵������ȷ���ǣ�������
| A�� | ����2һ����SO2 | B�� | ��ɫ����2һ����BaSO4 | ||
| C�� | ��ɫ����1�ijɷ�ֻ��2�ֿ��� | D�� | ��Һ��ֻ��HCO3-��ȷ�� |

 ��
��