��Ŀ����
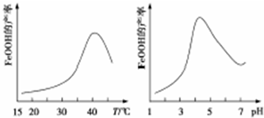
12�������£���0.02mol•L-1MOH��Һ�ζ�100mL0.01mol•L-1HA��Һ����ͼΪ����MOH��Һ�����������Һ��pH�仯�������Һ����仯���Բ��ƣ����ش��������⣺��1����ͼ����Ϣ��֪HAΪǿ�ᣨ�ǿ�������������ζ����õ�������ָʾ��Ϊ���ȣ��ζ�ʱ�۾�ע����ƿ����ɫ�仯��
��2��������һ��Ũ�ȵ�MAϡ��Һ��pH=a����a��7������ڡ�����С�ڡ����ڡ�����ʱ����Һ��ˮ�������c��OH-��=10-amol/L��
��3����д��K������Ӧ����Һ������Ũ�ȵ��ɴ�С�Ĺ�ϵ��c��M+����c��A-����c��OH-����c��H+����
��4��K������Ӧ����Һ�У�c��M+��+c��MOH������2c��A-����������ڡ���С�ڡ������ڡ���
��5��������MOH��Һ��ˮϡ�����У�������ֵ��С���Ǣۣ�����ţ�
��c��H+�� ��$\frac{C��{H}^{+}��}{C��O{H}^{-}��}$ ��c��OH-�� ��$\frac{C��O{H}^{-}��}{C��{M}^{+}��}$��

���� ��1������ͼ֪��δ�Ӽ�ʱ0.01mol•L-1HA��Һ��pH=2��˵����Һ��c��H+��=c��HA����HA��ȫ���룻
�������Һ��pH=7ʱ��n��HA����n��MOH����˵��MOH���ֵ���Ϊ�������ǡ����ȫ�к�ʱ���ɵ�MA��ǿ�������Σ�����Һ�����ԣ��ζ�ʱ�۾�Ҫע�ӻ����Һ��ɫ�仯��
��2��MA��ǿ�������Σ���������ˮ��¸���Һ�����ԣ�ˮ�������c��OH-�����ڸ���Һ��c��H+����
��3��K��n��MOH��=2n��HA������Һ�е�����Ϊ�����ʵ���Ũ�ȵ�MOH��MA��MOH����̶ȴ���MAˮ��̶ȵ�����Һ�ʼ��ԣ���ϵ���غ��ж�����Ũ�ȴ�С��
��4��K��n��MOH��=2n��HA������Һ�д��������غ㣬���������غ��жϣ�
��5��MOH��Һ��ˮϡ�ʹٽ�MOH���룬��MOH��������̶�С����Һ�������̶ȣ�����Һ��c��OH-����c��MOH����c��M+������С��
��� �⣺��1������ͼ֪��δ�Ӽ�ʱ0.01mol•L-1HA��Һ��pH=2��˵����Һ��c��H+��=c��HA����HA��ȫ����Ϊǿ�
�������Һ��pH=7ʱ��n��HA����n��MOH����˵��MOH���ֵ���Ϊ�������ǡ����ȫ�к�ʱ���ɵ�MA��ǿ�������Σ�����Һ�����ԣ�������Ҫѡȡ����Ϊָʾ�����ζ�ʱ�۾�Ҫע�ӻ����Һ��ɫ�仯�������۾�Ҫע����ƿ����ɫ�仯��
�ʴ�Ϊ��ǿ�����ȣ���ƿ����ɫ�仯��
��2��MA��ǿ�������Σ���������ˮ��¸���Һ�����ԣ�����a��7��ˮ�������c��OH-�����ڸ���Һ��c��H+��Ϊ10-a mol/L��
�ʴ�Ϊ������10-a��
��3��K��n��MOH��=2n��HA������Һ�е�����Ϊ�����ʵ���Ũ�ȵ�MOH��MA��MOH����̶ȴ���MAˮ��̶ȵ�����Һ�ʼ��ԣ���c��OH-����c��H+������ϵ���غ��c��M+����c��A-����MOH���������������Һ������Ũ�ȴ�С˳����c��M+����c��A-����c��OH-����c��H+����
�ʴ�Ϊ��c��M+����c��A-����c��OH-����c��H+����
��4��K��n��MOH��=2n��HA������Һ�д��������غ㣬���������غ��c��M+��+c��MOH������2c��A-����
�ʴ�Ϊ�����ڣ�
��5��MOH��Һ��ˮϡ�ʹٽ�MOH���룬��MOH��������̶�С����Һ�������̶ȣ�����Һ��c��OH-����c��MOH����c��M+������С��
��c��OH-����С�����ӻ��������䣬��c��H+�����ʴ���
��ϡ������c��OH-����С��$\frac{C��{H}^{+}��}{C��O{H}^{-}��}$=$\frac{{K}_{w}}{{c}^{2}��O{H}^{-}��}$���ʴ���
��ϡ�����дٽ�MOH���룬�����������̶�С����Һ�������̶ȣ�����c��OH-����С������ȷ��
��ϡ������c��OH-����c��M+����С�̶���ͬ����$\frac{C��O{H}^{-}��}{C��{M}^{+}��}$���䣬�ʴ���
�ʴ�Ϊ���ۣ�
���� ���⿼���������Һ�����жϣ�Ϊ��Ƶ���㣬���ؿ���ѧ�������ж���������ȷ�ζ���������Һ�е����ʳɷּ��������ǽⱾ��ؼ���ע�⣺ϡ�����������ֻ��c��H+������������Ũ�ȶ���С��

 ��У����ϵ�д�
��У����ϵ�д�| A�� | ��Ҫ���Ȳ��ܷ����ķ�Ӧ��һ�������ȷ�Ӧ | |
| B�� | 1mol�����ǡ���к�ʱ���ų����������к��� | |
| C�� | ��ѧ��Ӧ�е������仯������Ϊ�����ı仯 | |
| D�� | ��ѧ��Ӧ�е������仯����Ҫ���ɻ�ѧ���ı仯����� |
 X��Y��ZΪԭ��������������Ķ���������Ԫ�أ�����Ԫ�����ڲ�ͬ���ڣ�����ת����ϵ�У�A��B��C��X��Y��Z��Ӧ��������̬���ʣ������Ϊ������������з�����ȷ���ǣ�������
X��Y��ZΪԭ��������������Ķ���������Ԫ�أ�����Ԫ�����ڲ�ͬ���ڣ�����ת����ϵ�У�A��B��C��X��Y��Z��Ӧ��������̬���ʣ������Ϊ������������з�����ȷ���ǣ�������| A�� | ���Ӱ뾶��Y��Z | B�� | Z�ĺ������Ϊǿ�� | ||
| C�� | ��Yͬ�����軯����D���ȶ� | D�� | F�����Ӽ����ۼ� |
 X��Y��Z��WΪԭ������������4�ֶ�������Ԫ�أ�����Y��ZΪ����Ԫ�أ�X��Y��Z��W������������Ӧ��ˮ����ס��ҡ�������֮���������ͼ��ʾ��Ӧ��ϵ��ͼ�С�һ�����������������ܷ�����Ӧ���������ж���ȷ���ǣ�������
X��Y��Z��WΪԭ������������4�ֶ�������Ԫ�أ�����Y��ZΪ����Ԫ�أ�X��Y��Z��W������������Ӧ��ˮ����ס��ҡ�������֮���������ͼ��ʾ��Ӧ��ϵ��ͼ�С�һ�����������������ܷ�����Ӧ���������ж���ȷ���ǣ�������| A�� | X��Ԫ�����ڱ��зǽ�������ǿ��Ԫ�� | |
| B�� | Zλ��Ԫ�����ڱ���3����IA�� | |
| C�� | 4��ԭ���У�Yԭ�Ӱ뾶��С | |
| D�� | W�������ӿ��ܴٽ�ˮ�ĵ��� |

| A�� | ���ڷ����� | B�� | �Ǹ߷��ӻ����� | ||
| C�� | ��ʹ��ˮ�����Ը��������Һ��ɫ | D�� | �����ϵ�һ��ȡ������5�� |