��Ŀ����
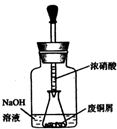 ��ʽ̼��ͭ Cu2��OH��2CO3��һ����;�㷺�Ļ���ԭ�ϣ�ʵ�����Է�ͭмΪԭ����ȡ��ʽ̼��ͭ�IJ������£�
��ʽ̼��ͭ Cu2��OH��2CO3��һ����;�㷺�Ļ���ԭ�ϣ�ʵ�����Է�ͭмΪԭ����ȡ��ʽ̼��ͭ�IJ������£�����һ����ͭм������ͭ
��ͼ���ý�ͷ�ι���ȡŨHNO3�����ӵ���ƿ�ڵķ�ͭм�У���ͭм����������ַ�Ӧ����ˣ��õ�����ͭ��Һ��
���������ʽ̼��ͭ���Ʊ�
����Թ��м���̼������Һ������ͭ��Һ��ˮԡ������70�����ң���0.4mol/L��NaOH��Һ����pH��8.5�������ã����ˣ�����ˮϴ�ӣ���ɣ��õ���ʽ̼��ͭ��Ʒ��
���������գ�
��1��д��Ũ������ͭ��Ӧ�����ӷ���ʽ
��2����ͼװ����NaOH��Һ��������
��3��������У�ˮԡ��������������
��4�����������Һ�п��ܺ���CO32-��д������CO32-�ķ���
��5��Ӱ���Ʒ��������Ҫ������
��6����ʵ��õ�2.42g��Ʒ��ֻ��CuO���ʣ���ȡ����Ʒ�������ֽ���ȫ�õ�1.80g���壬����Ʒ�м�ʽ̼��ͭ������������
���㣺�Ʊ�ʵ�鷽�������
ר�⣺
��������1��ͭ��Ũ���ᷴӦ��������ͭ������������ˮ��
��2����Ӧ���ɵĵ����������Ǵ�����Ⱦ���壬������������Һ���գ���ֹ��Ⱦ����NO2+NaOH��NaNO2+NaNO3+H2O����֪��Ӧ������ʣ���NaOH�⣬���������ɵ�NaNO2��NaNO3��
��3����Ӧ��Ϊ���Թܣ�ˮԡ�������ձ�ʢ��ˮ�����ڿ����¶ȣ����¶ȼƲⶨˮ�£���ʽ̼��ͭ���渽�ŵ������ƣ���Ҫϴ�ӳ�ȥ��
��4������̼������ᷴӦ�������壬�����ɵ�����ͨ�������ʯ��ˮ���м��飻
��5���ɲ������֪��ʵ��ɹ��Ĺؼ��ǿ��Ʒ�Ӧ�¶�����Һ��pHֵ��
��6����ʽ̼��ͭ��ȫ�ֽ�õ�CuO������Ʒ�м�ʽ̼��ͭ����������Ϊx�����ʽ̼��ͭ������Ϊ2.42x g�����ݼ�ʽ̼��ͭ�ֽⷽ��ʽ�����ò������з��̼��㣮
��2����Ӧ���ɵĵ����������Ǵ�����Ⱦ���壬������������Һ���գ���ֹ��Ⱦ����NO2+NaOH��NaNO2+NaNO3+H2O����֪��Ӧ������ʣ���NaOH�⣬���������ɵ�NaNO2��NaNO3��
��3����Ӧ��Ϊ���Թܣ�ˮԡ�������ձ�ʢ��ˮ�����ڿ����¶ȣ����¶ȼƲⶨˮ�£���ʽ̼��ͭ���渽�ŵ������ƣ���Ҫϴ�ӳ�ȥ��
��4������̼������ᷴӦ�������壬�����ɵ�����ͨ�������ʯ��ˮ���м��飻
��5���ɲ������֪��ʵ��ɹ��Ĺؼ��ǿ��Ʒ�Ӧ�¶�����Һ��pHֵ��
��6����ʽ̼��ͭ��ȫ�ֽ�õ�CuO������Ʒ�м�ʽ̼��ͭ����������Ϊx�����ʽ̼��ͭ������Ϊ2.42x g�����ݼ�ʽ̼��ͭ�ֽⷽ��ʽ�����ò������з��̼��㣮
���
�⣺��1��ͭ��Ũ���ᷴӦ��������ͭ������������ˮ����Ӧ���ӷ���ʽΪ��Cu+4H++2NO3-=Cu2++2NO2��+2H2O��
�ʴ�Ϊ��Cu+4H++2NO3-=Cu2++2NO2��+2H2O��
��2����Ӧ���ɵĵ����������Ǵ�����Ⱦ���壬������������Һ���գ���ֹ��Ⱦ����NO2+NaOH��NaNO2+NaNO3+H2O����֪��Ӧ������ʣ���NaOH�⣬���������ɵ�NaNO2��NaNO3��
�ʴ�Ϊ�����յ��������NaNO2��NaNO3��
��3����Ӧ��Ϊ���Թܣ�ˮԡ�������ձ�ʢ��ˮ�����ڿ����¶ȣ����¶ȼƲⶨˮ�£�ϴ�ӳ�ȥ��ʽ̼��ͭ���渽�ŵ������ƣ�
�ʴ�Ϊ���ձ����¶ȼƣ�ϴ�ӳ�ȥ��ʽ̼��ͭ���渽�ŵ������ƣ�
��4������̼����ķ���Ϊ��ȡ�����������ᣬ�����ɵ�����ͨ������ʯ��ˮ����Һ����ǣ�˵������CO32-��
�ʴ�Ϊ��ȡ�����������ᣬ�����ɵ�����ͨ������ʯ��ˮ����Һ����ǣ�˵������CO32-��
��5���ɲ������֪��ʵ��ɹ��Ĺؼ��ǿ��Ʒ�Ӧ�¶�����Һ��pHֵ����Ӱ���Ʒ��������Ҫ�����У���Ӧ�¶ȡ���ҺpHֵ��
�ʴ�Ϊ����Ӧ�¶ȣ���ҺpHֵ��
��6������Ʒ�м�ʽ̼��ͭ����������Ϊx����ʽ̼��ͭ������Ϊ2.42x g����
Cu2��OH��2CO3
2CuO+H2O+CO2�� ��������
222 62
2.42x g 2.42g-1.8g=0.62g
���ԣ�222��62=2.42x g��0.62g
���x=0.92
�ʴ�Ϊ��0.92��
�ʴ�Ϊ��Cu+4H++2NO3-=Cu2++2NO2��+2H2O��
��2����Ӧ���ɵĵ����������Ǵ�����Ⱦ���壬������������Һ���գ���ֹ��Ⱦ����NO2+NaOH��NaNO2+NaNO3+H2O����֪��Ӧ������ʣ���NaOH�⣬���������ɵ�NaNO2��NaNO3��
�ʴ�Ϊ�����յ��������NaNO2��NaNO3��
��3����Ӧ��Ϊ���Թܣ�ˮԡ�������ձ�ʢ��ˮ�����ڿ����¶ȣ����¶ȼƲⶨˮ�£�ϴ�ӳ�ȥ��ʽ̼��ͭ���渽�ŵ������ƣ�
�ʴ�Ϊ���ձ����¶ȼƣ�ϴ�ӳ�ȥ��ʽ̼��ͭ���渽�ŵ������ƣ�
��4������̼����ķ���Ϊ��ȡ�����������ᣬ�����ɵ�����ͨ������ʯ��ˮ����Һ����ǣ�˵������CO32-��
�ʴ�Ϊ��ȡ�����������ᣬ�����ɵ�����ͨ������ʯ��ˮ����Һ����ǣ�˵������CO32-��
��5���ɲ������֪��ʵ��ɹ��Ĺؼ��ǿ��Ʒ�Ӧ�¶�����Һ��pHֵ����Ӱ���Ʒ��������Ҫ�����У���Ӧ�¶ȡ���ҺpHֵ��
�ʴ�Ϊ����Ӧ�¶ȣ���ҺpHֵ��
��6������Ʒ�м�ʽ̼��ͭ����������Ϊx����ʽ̼��ͭ������Ϊ2.42x g����
Cu2��OH��2CO3
| ||
222 62
2.42x g 2.42g-1.8g=0.62g
���ԣ�222��62=2.42x g��0.62g
���x=0.92
�ʴ�Ϊ��0.92��
���������⿼�������Ʊ�ʵ�飬�ۺϿ���ѧ����ʵ����������Ҫѧ���߱���ʵ�Ļ������Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ



