��Ŀ����
5��ӡȾ��ҵ�����������ƣ�NaClO2��Ư��֯�Ư��֯��ʱ���������õ���HClO2���±���25��ʱHClO2 �����ֳ�������ĵ���ƽ�ⳣ����| ���� | HClO2 | HF | HCN | H2S |
| Ka | 1��10-2 | 6.3��10-4 | 4.9��10-10 | K1=9.1��10-8K2=1.1��10-12 |
��2��Na2S�dz��õij�������ij��ҵ��ˮ�к��е�Ũ�ȵ�Cu2+��Fe2+��Pb2+ ���ӣ��μ�Na2S��Һ�����������ij�����CuS�������һ�����ӳ�����ȫʱ��������Ũ��Ϊ10-5mol•L-1����ʱ��S2-��Ũ��Ϊ6.3��10-13��
��֪Ksp��FeS��=6.3��10-18��mol•L-1��2��Ksp��CuS��=6��10-36��mol•L-1��2Ksp��PbS��=2.4��10-28��mol•L-1��2��
���� ��1������ĵ���ƽ�ⳣ��Խ������Խǿ����֮����Խ��������Խ������Ӧ�������������ˮ��̶�Խ����Һ��PHԽ��
NaF��NaCN����Һ��������Ũ����ͬ�����������Ӷ���-1�����ӣ�1mol������ˮ��õ�1mol���������ӣ������ӵ���Ũ�Ȳ��䣬����Һ����������Ũ����ͬ������Һ����Խǿ��������Ũ��ԽС��������������������ԽС��
��2���������������ͬ���ܶȻ�ԽС���ܽ��ԽС���μ����ƣ���Ӧ���������ȳ����������ܶȻ�����S2-��Ũ�ȣ�
��� �⣨1�����ݵ���ƽ�ⳣ����֪����ǿ��˳��Ϊ��HClO2��HF��HCN��HS-������Խ������Ӧ�������������ˮ��̶�Խ����Һ��PHԽ�����ʵ���Ũ����ȸ���ҺpH��ϵΪ��pH��Na2S����pH��NaCN����pH��NaF����pH��NaClO2����
NaF��NaCN����Һ��������Ũ����ͬ�����������Ӷ���-1�����ӣ�1mol������ˮ��õ�1mol���������ӣ������ӵ���Ũ�Ȳ��䣬����Һ����������Ũ����ͬ����ҺΪ���ԣ�pH��NaCN����pH��NaF������NaCN��Һ��������Ũ�Ƚ�С��������������������С����NaF��Һ���������������ϴ�
�ʴ�Ϊ��pH��Na2S����pH��NaCN����pH��NaF����pH��NaClO2����ǰ�ߴ�
��2���������������ͬ���ܶȻ�ԽС���ܽ��ԽС���μ����ƣ���Ӧ���������ȳ���������������������CuS��
Fe2+��������������ȫʱ��Ũ��Ϊ10-5mol•L-1����ʱ��S2-��Ũ��Ϊ$\frac{6.3��1{0}^{-18}}{1{0}^{-5}}$mol/L=6.3��10-13mol/L��
�ʴ�Ϊ��CuS��6.3��10-13mol/L��
���� ���⿼���˵��볣����Ӧ�á��ε�ˮ�⡢�ܶȻ����йؼ�����Ӧ�õȣ���Ŀ�Ѷ��еȣ������ڿ���ѧ���Ķ���Ŀ��ȡ��Ϣ�������ͼ���������

| A�� | ��һ���¶ȣ���ͬŨ��ʱ����ƽ�ⳣ����K��Խ����Ա�ʾ������ʵ���̶�Խ�� | |
| B�� | ����ƽ�ⳣ����K�����¶��� | |
| C�� | ��ͬŨ�ȵ�ͬһ������ʣ������ƽ�ⳣ����K����ͬ | |
| D�� | ��Ԫ�����������ƽ�ⳣ�����ϵΪK1��K2��K3 |
| ��ѧʽ | CH3COOH | H2CO3 | HClO |
| ����ƽ�ⳣ�� | 1.7��10-5 | K1=4.3��10-7 K2=5.6��10-11 | 3.0��10-8 |
��1��CH3COOH��H2CO3��HClO��������ǿ������˳��ΪCH3COOH��H2CO3��HClO��
��2��ͬŨ�ȵ�CH3COO-��HCO3-��CO32-��ClO-���H+��������ǿ������˳��ΪCO32-��ClO-��HCO3-��CH3COO-��
��3��ͬŨ�ȵĴ����ơ��������ơ�̼����������Һ��pHֵ��С�����˳��ΪNa2CO3��NaClO��CH3COONa��
 ����Al��OH��3 ��Ksp=1.3��10-33��Mg��OH�� 2��Ksp=5.6��10-12�������ʵ���Ũ��AlCl3��MgCl2�����Һ��NaOH��Һ��Ӧ������Ӧ������pH���������ϵ����ͼ��
����Al��OH��3 ��Ksp=1.3��10-33��Mg��OH�� 2��Ksp=5.6��10-12�������ʵ���Ũ��AlCl3��MgCl2�����Һ��NaOH��Һ��Ӧ������Ӧ������pH���������ϵ����ͼ�������й�˵����ȷ���ǣ�������
| A�� | Al��OH��3 ��Mg��OH��2��ˮ��Һ�еĵ��������ȫ��ͬ | |
| B�� | ���ı���Һ��pH����pH��4.7ʱ��n��Mg2+���������仯 | |
| C�� | ��pH��11.1ʱ����Һ��ֻ��NaCl��NaAlO2��Na[Al��OH��4]�� | |
| D�� | �����ʵ���Ũ��Al3+��Mg2+�����Һ�백ˮ��Ӧ������Ӧ��������ˮ��pH���������ϵ����ͼ���� |
| A�� | H2SO4�TH22++SO42- | B�� | NaHCO3�TNa++H++CO32- | ||
| C�� | Ba��OH��2�TBa2++2��OH��- | D�� | Na3PO4�T3Na++PO43- |
| ���� | ���� | �����Լ� | ��Ҫ���� | |
| A | SiO2 | Fe2O3 | ���� | ���� |
| B | Mg | Al | NaOH��Һ | ���� |
| C | FeCl2 | FeCl3 | Cu | ���� |
| D | NaCl | ��ɳ | ��ˮ | �ܽ⡢���ˡ����� |
| A�� | A | B�� | B | C�� | C | D�� | D |
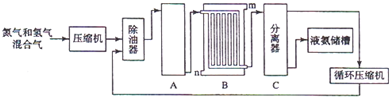
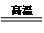 H2+CO��CH4+H2O
H2+CO��CH4+H2O 3H2+CO��
3H2+CO��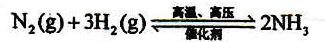 ��
��