��Ŀ����
 ��1�������£������0.1mol NH4Cl��0.05mol NaOHȫ������ˮ���γɻ����Һ����������ʧ����
��1�������£������0.1mol NH4Cl��0.05mol NaOHȫ������ˮ���γɻ����Һ����������ʧ������
��
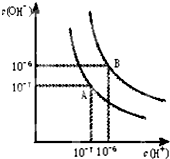
��2��ˮ�ĵ���ƽ��������ͼ��ʾ��
������A���ʾ25��ʱˮ�ڵ���ƽ��ʱ������Ũ�ȣ����¶�����100��ʱ��ˮ�ĵ���ƽ��״̬��B�㣬���ʱˮ�����ӻ���
�ڽ�100���pH=8��Ba��OH��2��Һ��100���pH=5��ϡ�����ϣ�������100��ĺ��£���ʹ�����ҺpH=7����Ba��OH��2������������Ϊ
���㣺�����ʱ�Ķ����жϼ��й�ph�ļ���,ˮ�ĵ���,����ˮ���Ӧ��
ר�⣺����ƽ������Һ��pHר��,�����ˮ��ר��
��������1���ٶ����ʱ������ӦNH4Cl+NaOH=NH3��H2O+NaCl������Һ�е�����Ϊ�����ʵ���Ũ�ȵ��Ȼ��ơ��Ȼ�狀Ͱ�ˮ����Һ�д��������غ㣬���������غ��жϣ�
����Һ�д��ڵ���غ㣬���ݵ���غ��жϣ�
��2����ˮ�ĵ��������ȷ�Ӧ�������¶ȴٽ�ˮ���룬��ˮ��c��H+����10-7 mol/L����10-6mol/L����ˮ��c��H+��=c��OH-����c��H+��?c��OH-��=Kw��
��100���pH=8��Ba��OH��2��Һ��c��OH-��=
mol/L=10-4mol/L��100���pH=5��ϡ������Һ��c��H+��=10-5 mol/L������100��ĺ��£������ҺpH=7��������Һ��c��OH-��=
mol/L=10-5mol/L����Һ�ʼ��ԣ�
������������Һ���ΪxL��ϡ�������ΪyL������c��OH-��=
=10-5mol/L���ݴ˼�������������ϡ�������֮�ȣ�
����Һ�д��ڵ���غ㣬���ݵ���غ��жϣ�
��2����ˮ�ĵ��������ȷ�Ӧ�������¶ȴٽ�ˮ���룬��ˮ��c��H+����10-7 mol/L����10-6mol/L����ˮ��c��H+��=c��OH-����c��H+��?c��OH-��=Kw��
��100���pH=8��Ba��OH��2��Һ��c��OH-��=
| 10-12 |
| 10-8 |
| 10-12 |
| 10-7 |
������������Һ���ΪxL��ϡ�������ΪyL������c��OH-��=
| 10-4mol/L��xL-10-5mol/L��yL |
| (x+y)L |
���
�⣺��1���ٶ����ʱ������ӦNH4Cl+NaOH=NH3��H2O+NaCl������Һ�е�����Ϊ�����ʵ���Ũ�ȵ��Ȼ��ơ��Ȼ�狀Ͱ�ˮ����Һ�д��������غ㣬���������غ��n��NH4+��+n��NH3��H2O��=0.1mol��
�ʴ�Ϊ��NH4+��NH3��H2O��
����Һ�д��ڵ���غ㣬���ݵ���غ��n��H+��+n��NH4+��+n��Na+��=n��Cl-��+n��OH-���������Ӻ������Ӳ�ˮ�⣬����n��Na+��=0.05mol��n��Cl-��=0.1mol����n��H+��+n��NH4+��-n��OH-��=n��Cl-��-n��Na+��=0.1mol-0.05mol=0.05mol��
�ʴ�Ϊ��H+��NH4+��
��2����ˮ�ĵ��������ȷ�Ӧ�������¶ȴٽ�ˮ���룬��ˮ��c��H+����10-7 mol/L����10-6mol/L����ˮ��c��H+��=c��OH-����c��H+��?c��OH-��=Kw������25��ʱˮ�����ӻ�����Ϊ10-14��100��ʱˮ�����ӻ�����Ϊ10-12��
�ʴ�Ϊ��10-14��10-12��
��100���pH=8��Ba��OH��2��Һ��c��OH-��=
mol/L=10-4mol/L��100���pH=5��ϡ������Һ��c��H+��=10-5 mol/L������100��ĺ��£������ҺpH=7������Ϻ���Һ�ʼ��ԣ�������������Һ���Ϊx��ϡ�������Ϊy��c��OH-��=
=10-5mol/L��x��y=2��9��
�ʴ�Ϊ��2��9��
�ʴ�Ϊ��NH4+��NH3��H2O��
����Һ�д��ڵ���غ㣬���ݵ���غ��n��H+��+n��NH4+��+n��Na+��=n��Cl-��+n��OH-���������Ӻ������Ӳ�ˮ�⣬����n��Na+��=0.05mol��n��Cl-��=0.1mol����n��H+��+n��NH4+��-n��OH-��=n��Cl-��-n��Na+��=0.1mol-0.05mol=0.05mol��
�ʴ�Ϊ��H+��NH4+��
��2����ˮ�ĵ��������ȷ�Ӧ�������¶ȴٽ�ˮ���룬��ˮ��c��H+����10-7 mol/L����10-6mol/L����ˮ��c��H+��=c��OH-����c��H+��?c��OH-��=Kw������25��ʱˮ�����ӻ�����Ϊ10-14��100��ʱˮ�����ӻ�����Ϊ10-12��
�ʴ�Ϊ��10-14��10-12��
��100���pH=8��Ba��OH��2��Һ��c��OH-��=
| 10-12 |
| 10-8 |
| 10-4mol/L��x-10-5mol/L��y |
| x+y |
�ʴ�Ϊ��2��9��
���������⿼�����������Һ�����жϡ�ˮ�ĵ��롢����ˮ���֪ʶ�㣬��1������ȷ��Һ�е������ǽⱾ��ؼ�������غ�˼��������ע�⣨2����100��ʱpH=7����Һ�������Զ��dzʼ��ԣ�Ϊ�״��㣮

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
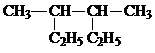 ���ƣ�
���ƣ�