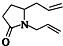
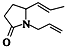
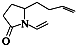
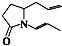
��Ŀ����
2��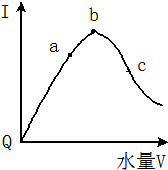 �������£��������ˮϡ�����У���Һ��������I�����ˮ����V��ʾ������ı仯������ͼ��ʾ����ش�
�������£��������ˮϡ�����У���Һ��������I�����ˮ����V��ʾ������ı仯������ͼ��ʾ����ش���1����Q���㵼����������ԭ������Ϊ������δ���룬�������ƶ������ӣ�
��2��a��b��c���㴦����Һ��C��H+����С�����˳����c��a��b������̶���С�����˳����a��b��c��
��3����ҪʹC�㴦��Һ��C��CH3COO-������C��H+����С���ɲ�ȡ�Ĵ�ʩ�ǣ�������������ι��壬���ý��������������̼���εȣ�
���� ��1����Һ�ĵ�����������Ũ���йأ�����Ũ��Խ������Խǿ��
��2����������Խǿ������Ũ��Խ��������Ũ��Խ��pHԽС����ҺԽϡ��Խ�ٽ�������룻
��3��Ҫʹ���������Ũ�������Բ��ü��ȡ����뺬�д�������ӵ����ʡ�����������ӷ�Ӧ�����ʣ�
��� �⣺��1����Һ�ĵ�����������Ũ���йأ�����Ũ��Խ������Խǿ����������û�������ƶ������ӣ����Ա�������磬
�ʴ�Ϊ����Ϊ������δ���룬�������ƶ������ӣ�
��2����������Խǿ������Ũ��Խ��������Ũ��Խ����a��b��c������Һ��������Ũ����С�����˳��ΪΪc��a��b����ҺԽϡ��Խ�ٽ�������룬����Һ�������ӵ����ʵ���Խ����̶�Խ�ʴ�Ϊ��c��a��b��a��b��c��
��3����NaOH���壬�������ƺ������ӷ�Ӧ�ٽ�������룬���Դ��������Ũ�����ӹ���CH3COONa�������ƴ�����룬�������Ƶ�����Ĵ�������Ӵ������ƴ��������Ĵ�������ӣ����Դ��������Ũ��������п�����������ӷ�Ӧ���ٽ�������룬���Դ��������Ũ������MgO���壬�������ӷ�Ӧ���ٽ�������룬��Na2CO3�����������ӷ�Ӧ���ٽ�������룬
�ʴ�Ϊ��������������ι��壬���ý��������������̼���εȣ�
���� �����ۺϿ������ʵĵ��룬������ѧ���ķ��������Ŀ��飬Ϊ�߿��������ͺ�Ƶ���㣬��Ŀ�ѶȲ���

| A�� | BeCl2Ϊ���ۻ����� | |
| B�� | At2Ϊ��ɫ���壬HAt���ȶ���AgAt�й���ǿ����������ˮҲ������ϡ�� | |
| C�� | ���ᱵ��������ˮ�İ�ɫ���� | |
| D�� | ����������ɫ���ж�����H2S�ȶ������� |
| ѡ�� | ������ | ������ |
| A | ����ˮ��ʱ�������������ԵĽ������� | ������������ˮ���� |
| B | Fe3+�������� | FeCl3��Һ�������ܽ���վɵ�·�� �е�ͭ |
| C | NH4Cl���ȷֽ� | ���ȿɽ�Ca��OH��2��NH4Cl����������� |
| D | SO2���������� | SO2������Ư��ֽ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 1 | ⑬ | |||||||
| 2 | �� | ⑭ | �� | |||||
| 3 | �� | �� | �� | �� | �� | �� | �� | |
| 4 | �� | ⑪ | ⑫ |
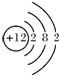
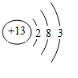
��2������ԭ�ӵĽṹʾ��ͼ����
 ����
���� ����
����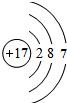 ��
����3������ЩԪ���У�����õĽ���Ԫ����K������õķǽ���Ԫ����F������õ�Ԫ����Ar��
��4������ЩԪ�ص�����������Ӧˮ�����У�������ǿ����HClO4��������ǿ����KOH�������Ե�����������Al��OH��3��
��5���ɱ���Ԫ���γɵij�������X��Y��Z��M��N�ɷ������·�Ӧ��
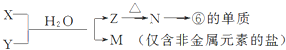
X��Һ��Y��Һ��Ӧ�����ӷ���ʽΪAl3++3NH3•H2O�TAl��OH��3��+3NH${\;}_{4}^{+}$��
N���ĵ��ʵĻ�ѧ����ʽΪ2Al2O3�����ڣ�$\frac{\underline{\;\;\;���\;\;\;}}{����ʯ}$4Al+3O2����
��6���ڢ�����У���ѧ���ʽϻ��õ���Na�������û�ѧʵ��֤����
����ˮ��Ӧ��
�ڢ���⑫�У���ѧ���ʽϻ��õ���Cl2�����û�ѧ����ʽ��˵����
��Cl2+2NaBr=2NaCl+Br2��
| A�� | ��$\underset{\stackrel{16}{\;}}{8}{O}_{2}$��Ϊͬλ�� | B�� | ������������ͬ�Ļ�ѧ���� | ||
| C�� | ��������Ϊͬ�������� | D�� | ��ͬ����������������ͬ����� |
 �̶�������CO2������Ч��������Դ�������ٿ����е��������壮��ҵ�������о�����CO2�������״�ȼ�ϵķ������÷����Ļ�ѧ����ʽ�ǣ�CO2��g��+3H2��g��?CH3OH��g��+H2O��g����H=-49.0kJ•mol-1
�̶�������CO2������Ч��������Դ�������ٿ����е��������壮��ҵ�������о�����CO2�������״�ȼ�ϵķ������÷����Ļ�ѧ����ʽ�ǣ�CO2��g��+3H2��g��?CH3OH��g��+H2O��g����H=-49.0kJ•mol-1
 ���ǣ�������
���ǣ�������