��Ŀ����
 1Lij�����Һ�����ܺ��е��������±���
1Lij�����Һ�����ܺ��е��������±���| ���ܴ������е������� | H+NH4+Al3+K+ |
| ���ܴ������е������� | Cl-Br-I?ClO?AlO2- |
�����Һ��ȷ�����е�������
����ȷ���Ƿ��е���������
�϶������ڵ���������
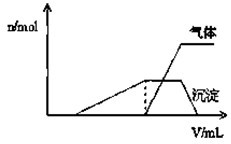
��2������⣬����Һ�к��д�����Cl-��Br-��I-������1L�û����Һ��ͨ��һ������Cl2����Һ��Cl-��Br-��I-�����ʵ�����ͨ��Cl2�������״�����Ĺ�ϵ���±���ʾ��������ش��������⣺
| Cl2���������״���� | 2.8L | 5.6L | 11.2L |
| n��Cl-�� | 1.25mol | 1.5mol | 2mol |
| n��Br-�� | 1.5mol | 1.4mol | 0.9mol |
| n��I-�� | a mol | 0 | 0 |
��ԭ��Һ��Cl-��Br-��I-�����ʵ���Ũ��֮��Ϊ
���㣺���������ӵļ���,���������ӵļ���
ר�⣺���ӷ�Ӧר��
��������1�����ݵ�һ�Σ�û�����ɳ�����˵��һ�����������ӣ��ʿ϶�������ClO?��AlO2-�����ɳ����ں�����ȫ�ܽ⣬˵��һ�����������ӣ��϶�������Mg2+��Fe3+��Cu2+�����ݵ����Σ����������Ʒ�Ӧ������������˵��һ������笠����ӣ���ԭ��Һ�к��е���������H+��NH4+��Al3+���϶�������Mg2+��Fe3+��Cu2+��ClO?��AlO2-�����ܺ���K+��Cl-��Br-��I-����2����n��Cl2��=
=0.125mol��ͨ������������I-��Ӧ�����ɵ��ʵ⣬����ʽΪ��Cl2+2I-=I2+2Cl-��
��2.8Lʱ��n��Br-��=1.5mol����ԭ��Һ��n��Br-��=1.5mol��
n��Cl-��=0.125��2=0.25mol��n��I-��=0.25mol����ԭ��Һ��n��Cl-��=1.25-0.25=1mol��
5.6Lʱ��n��Br-��=1.5-1.4=0.1mol��n��I-��=0.25-0.1=0.15����ԭ��Һ��n��I-��=0.25+0.15=0.4mol��
| 2.8 |
| 22.4 |
��2.8Lʱ��n��Br-��=1.5mol����ԭ��Һ��n��Br-��=1.5mol��
n��Cl-��=0.125��2=0.25mol��n��I-��=0.25mol����ԭ��Һ��n��Cl-��=1.25-0.25=1mol��
5.6Lʱ��n��Br-��=1.5-1.4=0.1mol��n��I-��=0.25-0.1=0.15����ԭ��Һ��n��I-��=0.25+0.15=0.4mol��
���
�⣺��1�����ݵ�һ�Σ�û�����ɳ�����˵��һ�����������ӣ��ʿ϶�������ClO?��AlO2-�����ɳ����ں�����ȫ�ܽ⣬˵��һ�����������ӣ��϶�������Mg2+��Fe3+��Cu2+�����ݵ����Σ����������Ʒ�Ӧ������������˵��һ������笠����ӣ���ԭ��Һ�к��е���������H+��NH4+��Al3+���϶�������Mg2+��Fe3+��Cu2+��ClO?��AlO2-�����ܺ���K+��Cl-��Br-��I-���ʴ�Ϊ��H+��NH4+��Al3+��K+��ClO?��AlO2-��
��2����n��Cl2��=
=0.125mol��ͨ������������I-��Ӧ�����ɵ��ʵ⣬����ʽΪ��Cl2+2I-=I2+2Cl-��
�ʴ�Ϊ��Cl2+2I-=I2+2Cl-��
��2.8Lʱ��n��Br-��=1.5mol����ԭ��Һ��n��Br-��=1.5mol��
n��Cl-��=0.125��2=0.25mol��n��I-��=0.25mol����ԭ��Һ��n��Cl-��=1.25-0.25=1mol��
5.6Lʱ��n��Br-��=1.5-1.4=0.1mol��n��I-��=0.25-0.1=0.15����ԭ��Һ��n��I-��=0.25+0.15=0.4mol��
��ԭ��Һ��Cl-��Br-��I-�����ʵ���Ũ��֮��Ϊ��1��1.5��0.4=10��15��4��
�ʴ�Ϊ��10��15��4��
��2����n��Cl2��=
| 2.8 |
| 22.4 |
�ʴ�Ϊ��Cl2+2I-=I2+2Cl-��
��2.8Lʱ��n��Br-��=1.5mol����ԭ��Һ��n��Br-��=1.5mol��
n��Cl-��=0.125��2=0.25mol��n��I-��=0.25mol����ԭ��Һ��n��Cl-��=1.25-0.25=1mol��
5.6Lʱ��n��Br-��=1.5-1.4=0.1mol��n��I-��=0.25-0.1=0.15����ԭ��Һ��n��I-��=0.25+0.15=0.4mol��
��ԭ��Һ��Cl-��Br-��I-�����ʵ���Ũ��֮��Ϊ��1��1.5��0.4=10��15��4��
�ʴ�Ϊ��10��15��4��
������������һ���й����Ӽ�����ۺ�֪ʶ��Ŀ������ǶȺܹ㣬�ѶȽϴ�

��ϰ��ϵ�д�
�����Ŀ
ʵ�����Ʊ���������ʱ�����÷�����ȷ���ǣ�������
| A��������ʱ����Na2O2��H2O2����Ӧ���ѡ����ͬ�����巢��װ�� |
| B��������ʱ���ñ���NaHCO3��Һ��Ũ���Ά������ |
| C������ϩʱ������ˮ���������ſ������ռ����� |
| D���ƶ�������ʱ����ˮ��NaOH��Һ����β�� |
ij���������ܺ���H2��CO��CO2��HCl��NH3��ˮ�����е����ֻ���֣��������������ͨ�����ٳ���ʯ��ˮ����������������������Һ���л���������ŨH2SO4������������������ͭ����죩������ˮ����ͭ����������������ÿһ�����վ���ȫ�����Ըû������ɷ��ж���ȷ���ǣ�������
| A��һ��û��CO2���϶���H2 |
| B��һ����CO��CO2��ˮ����? |
| C��һ����H2��CO2��HCl |
| D��������CO2��NH3��ˮ����? |
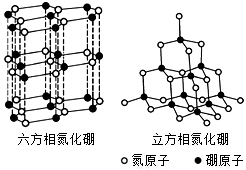 ������BN�������ж�����ṹ�������൪������ͨ�����ڵ��ȶ��࣬��ʯī���ƣ����в�״�ṹ�������������������൪�����dz�Ӳ���ϣ����������ĥ�ԣ����ǵľ���ṹ��ͼ��ʾ��
������BN�������ж�����ṹ�������൪������ͨ�����ڵ��ȶ��࣬��ʯī���ƣ����в�״�ṹ�������������������൪�����dz�Ӳ���ϣ����������ĥ�ԣ����ǵľ���ṹ��ͼ��ʾ��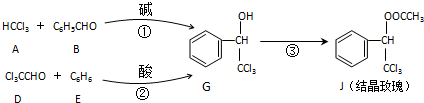
 +
+
 +H2O����Ӧ·�ߢڵõ�һ�ָ������˴Ź���������4��壬�������շ�����֮��Ϊ
+H2O����Ӧ·�ߢڵõ�һ�ָ������˴Ź���������4��壬�������շ�����֮��Ϊ
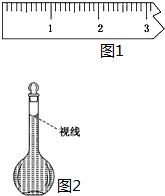 ʵ������Ҫ����0.50mol/L NaCl��Һ480mL�������в������������ʵ������֣���ʹ��������������
ʵ������Ҫ����0.50mol/L NaCl��Һ480mL�������в������������ʵ������֣���ʹ��������������