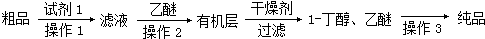��Ŀ����
14��ijУ��ѧ��ȤС���������к͵ζ����ⶨѧУ�¹�����ռ�Ĵ��ȣ��ռ��к��в����ᷴӦ�����ʣ����Ը���ʵ��ش���1��ʵ�鲽��Ϊ������������ƽȷ����4.1g�ռ���Ʒ��
�ڽ���Ʒ���250ml����Һ����Ҫ������������������Ͳ���ձ����Ҫ�IJ���������250ml����ƿ����ͷ�ιܣ�
���ü�ʽ�ζ�����ȡ10.00ml����Һ��ע����ƿ�У�
������ƿ�е���2��3�η�̪����ȣ���ָʾ��������0.2010mol/L�ı�����ζ������ռ���Һ���ζ�ʱ������ת��ʽ�ζ��ܵIJ������������ֲ�ͣ��ҡ����ƿ������ע����ƿ����Һ��ɫ�ı仯��ֱ���ζ��յ㣮
��2����������������
| �ζ����� | ����Һ���/ml | ���������/ml | |
| �ζ�ǰ����/ml | �ζ������/ml | ||
| ��һ�� | 10.00 | 0.20 | 22.90 |
| �ڶ��� | 10.00 | 0.50 | 20.40 |
| ������ | 10.00 | 4.00 | 24.10 |
| ���Ĵ� | 10.00 | 0.00 | 20.00 |
���� ��1�������250mL����Һ��һ����Ҫ250mL����ƿ����ͷ�ιܡ��ձ����������ȣ�
�۴���ҺΪ������Һ��
�ܿ����÷�̪�������ָʾ�����ζ�ʱ�����ֿ��ƻ������۾�ע����ƿ����ɫ�仯��
��2����������Ϊ$\frac{��22.90-0.20��+��20.40-0.50��+��24.10-4.00��+��20.00��}{4}$=20.675mL��20.68mL�����c��V��=c��V�����㣮
��� �⣺��1�������250mL����Һ��һ����Ҫ250mL����ƿ����ͷ�ιܡ��ձ����������ȣ��ʴ�Ϊ��250ml����ƿ����ͷ�ιܣ�
�۴���ҺΪ������Һ����ѡ��ȡ��ʽ�ζ��ܣ��ʴ�Ϊ����ʽ�ζ��ܣ�
����ζ�������÷�̪�������ָʾ�����ζ�ʱ�����ֿ�����ʽ�ζ��ܵĻ������۾�ע����ƿ����ɫ�仯��
�ʴ�Ϊ����̪����ȣ����ƿ����Һ��ɫ�ı仯��
��2����������Ϊ$\frac{��22.90-0.20��+��20.40-0.50��+��24.10-4.00��+��20.00��}{4}$=20.675mL��20.68mL����c��V��=c��V����֪��n��NaOH��=n��HCl��=20.68��0.001L��0.2010mol/L=0.0054mol���ռ�Ĵ���Ϊ$\frac{0.0054mol��\frac{250}{10}��40g/mol}{5.5g}$��100%=98%���ʴ�Ϊ��98%��
���� ���⿼�����ʵĺ����IJⶨ��Ϊ��Ƶ���㣬ע�ⷽ���IJ����ԡ������Է������к͵ζ�ʵ��ļ���Ϊ�����״��㣬ע�����ʵ���ԭ���Ͳ�������Ŀ�ѶȲ���

 ͨ��ѧ��Ĭд����ϵ�д�
ͨ��ѧ��Ĭд����ϵ�д� ij�¶��£�Fe��OH��3��s����Cu��OH��2��s���ֱ�����Һ�дﵽ�����ܽ�ƽ��ı���ҺpH������������Ũ�ȵı仯��ͼ��ʾ����ͼ�����������жϴ�����ǣ�������
ij�¶��£�Fe��OH��3��s����Cu��OH��2��s���ֱ�����Һ�дﵽ�����ܽ�ƽ��ı���ҺpH������������Ũ�ȵı仯��ͼ��ʾ����ͼ�����������жϴ�����ǣ�������| A�� | Ksp[Fe��OH��3]��Ksp[Cu��OH��2] | |
| B�� | Fe��OH��3��Cu��OH��2�ֱ���b��c�����������Һ�дﵽ���� | |
| C�� | ������NH4Cl�����ʹ��Һ��a��䵽b�� | |
| D�� | c��d�����������Һ��c��H+����c��OH-���˻���� |
| A�� | AgCl������ˮ������ת��ΪAgI | |
| B�� | �ں���Ũ�Ⱦ�Ϊ0.001 mol•L-1��Cl-��I-����Һ�л�������AgNO3ϡ��Һ����������AgI���� | |
| C�� | AgI��AgCl��������ˮ�����ԣ�AgCl����ת��ΪAgI | |
| D�� | �����£�AgCl��Ҫ��NaI��Һ�п�ʼת��ΪAgI����NaI��Ũ�ȱ��벻����$\frac{1}{\sqrt{1.8}}$��10-11mol•L-1 |

��10.0g��ϩ���6.0g�״�������������ƿ�У����Ӻ������ܣ��ý�������裬ˮԡ���ȣ�
�ڳ�ַ�Ӧ����ȴ������Һ�м���5% Na2CO3��Һϴ�����ԣ�
�۷�Һ��ȡ�ϲ���״Һ�壬������ˮNa2SO4����������ռ�70-90����֣�
�����õ�����Ϣ��
| �е� | �ܽ��� | ||
| ��ϩ�� | 141�� | ��ˮ���ܣ��������л��ܼ� | �ж� |
| �״� | 65�� | ��ˮ���ܣ��������л��ܼ� | �ӷ����ж� |
| ��ϩ���ϩ����� | 80.5�� | ������ˮ�������л��ܼ� | �ӷ� |
��1������c�������Ƿ�Һ©����
��2�����Һ��5%0Na2CO3��Һϴ�ӵ�Ŀ���dz�ȥ���Һ�еı�ϩ��ͼ״������ͱ�ϩ��������ܽ�ȣ���
��3����д������100g 5% Na2CO3��Һ��ʹ�õIJ��������ձ�������������Ͳ��
��4�����ڲ�Ʒ������������г�װ��δ����������ͼ����2��������ֱ�д���¶ȼ�ˮ����λ�á�β�ӹ�����ƿ�ӿ��ܷ⣮
Ϊ������ʣ��������ʵ�飺
�ٽ���״�����ᴿ��ƽ���ֳ�5�ݣ�ȡ��1��������ƿ�У�����2.5mol/L��KOH��Һ10-00mL������ʹ֮��ȫˮ�⣮
���÷�̪��ָʾ��������ȴ�����Һ�еμ�0.5mol/L��HCI��Һ���к�����KOH���ε��յ�ʱ����������20.00mL��
��5�����㱾��������Ӧ��ϩ���ת����54.0%��
��6�����о�2����ʵ������Ҫ��ȡ�İ�ȫ������ʩͨ�����ʵ�顢��ֹ����
| A�� | ��ԭ�� | B�� | ������ | C�� | ��ԭ���� | D�� | �������� |
| A�� | NaͶ�뵽ˮ�У�Na+H2O�TNa++OH-+H2�� | |
| B�� | AlCl3��Һ�м��������İ�ˮ��Al3++4OH-�TAlO2-+2H2O | |
| C�� | ���Ȼ�����Һ�м���ͭ�ۣ�Fe3++Cu�TFe2++Cu2+ | |
| D�� | ������ͨ���������������Һ�У�Cl2+2OH-�TCl-+ClO-+H2O |
 ʵ������ȡ�����������װ����ͼ��ʾ�������������������գ�
ʵ������ȡ�����������װ����ͼ��ʾ�������������������գ�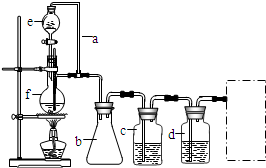 ijѧϰС���������кϳ�·�ߺϳ�1-������
ijѧϰС���������кϳ�·�ߺϳ�1-������