��Ŀ����
20����1�������й�������������˵����ȷ����A��CA����λ����ǿ���д���С�������е��������ȶ����еĺܲ��ȶ�
B��ˮ���ȵ��ܸߵ��¶ȶ����Էֽ�����Ϊˮ���Ӽ�������
C���ڻ����ڹ⻬��ǽ������������Ϊ�ڻ��ŵ�ϸë���������ķ��Ӳ������Ӽ�������
D����Ϊ�����칹���ķ��Ӿ�����ȫ��ͬ����ɺ�ԭ�����У�������������ҩ��ʱ������������칹��
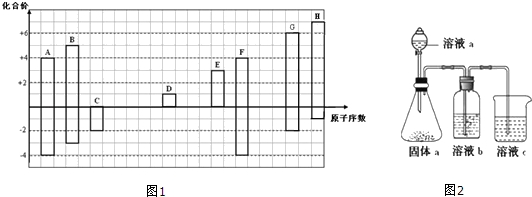
��2���ж������ĸ�ģ�ͣ�ͼ1��������������п�Ķѻ���ʽ�ң�

��3����������δ�ɶԵ���������P��Ԫ�ص�Ԫ�ط�����As���������ռ��18��ԭ�ӹ����
��4��̼��������������ӵ�ص��µ��Һ����Ҫ���Ӽ�����ṹ��ͼ2��̼ԭ�ӵ��ӻ���ʽ��sp3��sp2�������ЦҼ���м�֮��Ϊ10��1��
��5���Ƚ�NH2-��NH3�ļ��ǡ�HNH�Ĵ�С��NH2-��NH3���������=���������������ü۲���ӶԻ������ͣ�NH2-��Nԭ�ӹµ��Ӷ���Ϊ2��NH3��Nԭ�ӹµ��Ӷ���Ϊ1���µ�����ɼ����Ӽ�ij������ڳɼ�������ɼ����Ӽ�ij������¶Ե�����ǰ�߶࣬�ų�����ǿ������ǰ����С��
��6��SO32-����ԭ�ӵļ۲���ӶԻ���ģ���������Σ�д��һ����SO32-��Ϊ�ȵ�����ķ���PCl3����NF3��NCl3�������ɣ���
��7��SiC��������ʯ�������ƣ��辧���߳�Ϊa cm��̼ԭ��ֱ��Ϊb cm����ԭ��ֱ��Ϊc cm����þ����Ŀռ�������Ϊ$\frac{2�У�{b}^{3}+{c}^{3}��}{3{a}^{3}}��100%$��
���� ��1��A����λ����һ������Ĺ��ۼ��������ص����ڹ��õ�һ�Ե��ӳ���ͬһԭ�ӣ�����һ��ԭ���пչ����
B��ˮ���ȵ��ܸߵ��¶ȶ����Էֽ�����Ϊˮ���Ӽ�������
C���ڻ����ڹ⻬��ǽ������������Ϊ�ڻ��ŵ�ϸë���������ķ��Ӳ������Ӽ�������
D����Ϊ�����칹���ķ��Ӿ�����ȫ��ͬ����ɺ�ԭ�����У�������������ҩ��ʱ������������칹��
��2����Ϊ�������ѻ�����Ϊ�������ܶѻ�����Ϊ���������ѻ�����Ϊ�����������ܶѻ���
��3����������Ԫ���У���Χ�����Ų�Ϊndxnsy�����ܼ����ڰ����ȶ�״̬ʱ�����е�δ�ɶԵ�������࣬2������ռ��1��ԭ�ӹ����4p3���������ӷ�ռ3�����ݴ˽��
��4���÷���C-O��Cԭ�Ӽ۲���ӶԸ�����4�Ҳ����µ��Ӷԡ�C=O��Cԭ�Ӽ۲���ӶԸ�����3�Ҳ����µ��Ӷԣ����ݼ۲���ӶԻ��������ж�Cԭ���ӻ���ʽ��˫���к���һ���Ҽ���һ���м����������ǦҼ���
��5�����ݹ¶Ե�����ɼ����Ӽ�ij������ڳɼ�������ɼ����Ӽ�ij�����������
��6�����ݼ۲���ӶԻ�������ȷ����ռ乹�ͣ��۲���ӶԸ���=�Ҽ�����+�µ��ӶԸ������ݴ˷�����𣻾�����ͬԭ�����ͼ۵�������������Ϊ�ȵ����壻
��7�����ݾ�̯�����㾧����C��Siԭ����Ŀ���������㾧���к���C��Siԭ������������㾧��������������Ŀռ�������=$\frac{������C��Siԭ�������}{�������}$��100%��
��� �⣺��1��A�����ۼ���ǿ���д���С�����йµ��ӶԺͺ��пչ����ԭ��֮�����γ���λ������λ�����ڹ��ۼ��������е��������ȶ����еĺܲ��ȶ�����A��ȷ��
B��ˮ���ȵ��ܸߵ��¶ȶ����Էֽ�����Ϊˮ���Ӽ�������
C���ڻ����ڹ⻬��ǽ������������Ϊ�ڻ��ŵ�ϸë���������ķ��Ӳ������Ӽ�������
D����Ϊ�����칹���ķ��Ӿ�����ȫ��ͬ����ɺ�ԭ�����У�������������ҩ��ʱ������������칹��
�ʴ�Ϊ��A��C��
��2����������п�Ķѻ���ʽ�����������ܶѻ�������þ��п�����ͣ�Ϊͼʾ�ҵĶѻ���ʽ��
�ʴ�Ϊ���ң�
��3����������Ԫ���У���Χ�����Ų�Ϊndxnsy�����ܼ����ڰ����ȶ�״̬ʱ�����е�δ�ɶԵ�������࣬��������δ�ɶԵ���������P��Ԫ��Ϊ�飬��������Ų�ʽΪ��1s22s22p63s23p63d104s24p3��Ԫ�ط���ΪAs��2������ռ��1��ԭ�ӹ����4p3���������ӷ�ռ3�������Ժ������ռ��18��ԭ�ӹ����
�ʴ�Ϊ��As��18��
��4���÷���C-O��Cԭ�Ӽ۲���ӶԸ�����4�Ҳ����µ��Ӷԣ�Cԭ��Ϊsp3�ӻ���C=O��Cԭ�Ӽ۲���ӶԸ�����3�Ҳ����µ��Ӷԣ�Cԭ���ӻ���ʽΪsp2��˫���к���һ���Ҽ���һ���м����������ǦҼ������Ը÷����к���10���Ҽ���1���м������Է����ЦҼ���м�����֮��Ϊ10��1��
�ʴ�Ϊ��sp3��sp2��10��1��
��5���¶Ե�����ɼ����Ӽ�ij������ڳɼ�������ɼ����Ӽ�ij�����NH2-��Nԭ�ӹ¶Ե�����Ϊ2��NH3��Nԭ�ӹ¶Ե�����Ϊ1���¶Ե�����ǰ�߶࣬�ų�����ǿ������ǰ����С��
�ʴ�Ϊ������NH2-��Nԭ�ӹµ��Ӷ���Ϊ2��NH3��Nԭ�ӹµ��Ӷ���Ϊ1���µ�����ɼ����Ӽ�ij������ڳɼ�������ɼ����Ӽ�ij������¶Ե�����ǰ�߶࣬�ų�����ǿ������ǰ����С��
��6��SO32-����ԭ��Sԭ�ӹµ��Ӷ���=$\frac{6+2-3��2}{2}$=1�����Լ۲���Ӷ���=3+1=4����Sԭ�Ӳ�ȡsp3�ӻ���VSEPR����Ϊ�������壬��һ�Թ¶Ե��ӣ�Ϊ�����Σ��ȵ����壬����������ԭ�ӡ��۵�������ȣ�1molSO32-�к�4molԭ�ӣ���26mol�۵��ӵķ�����PCl3����NF3��NCl3�������ɣ���
�ʴ�Ϊ�������Σ�PCl3����NF3��NCl3�������ɣ���
��7�����ݾ�̯�����㾧����Cԭ����Ŀ=8��$\frac{1}{8}$+6��$\frac{1}{2}$=4��Siԭ����Ŀ=4�������߳�Ϊa cm�������Ϊa3cm3��̼ԭ��ֱ��Ϊb cm������Cԭ�������=4��$\frac{4}{3}$���С��� $\frac{1}{2}$b��3cm3=$\frac{2}{3}$��b3cm3����ԭ��ֱ��Ϊc cm������Siԭ�������=4��$\frac{4}{3}$���С��� $\frac{1}{2}$c��3cm3=$\frac{2}{3}$��c3cm3���ʾ�����C��Siԭ�������=$\frac{2}{3}$��b3cm3+$\frac{2}{3}$��c3cm3=$\frac{2}{3}$�У�b3+c3��cm3���ʾ����Ŀռ�������=$\frac{\frac{2}{3}�У�{b}^{3}+{c}^{3}��}{{a}^{3}}$��100%=$\frac{2�У�{b}^{3}+{c}^{3}��}{3{a}^{3}}��100%$��
�ʴ�Ϊ��$\frac{2�У�{b}^{3}+{c}^{3}��}{3{a}^{3}}��100%$��
���� �����Ƕ����ʽṹ�Ŀ��飬�漰��ѧ�����������������Ų����ɡ��ӻ��������������ȣ���7�����ݾ�̯�����㾧���Ǹ����ѵ㣬��Ҫѧ������һ���Ŀռ���������ѧ������������Ŀ�Ѷ��еȣ�


| X | Y | �������Һ | |
| A | Zn | Cu | ZnCl2��Һ |
| B | Cu | Zn | ϡH2SO4 |
| C | Cu | Zn | CuSO4��Һ |
| D | Zn | Zn | CuSO4��Һ |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ��ˮ�����Ҵ����������Ȼ�̼ | |
| B�� | �����Ը��������Һ���𱽡���ϩ�ͼ��� | |
| C�� | ��̼������Һ�����Ҵ���������������� | |
| D�� | ��ȼ�շ�������顢��ϩ����Ȳ |
| A�� | 1 mol��Ϊ2 g | B�� | H2O��Ħ������Ϊ18 g | ||
| C�� | 44 g CO2�����Ϊ22.4 L | D�� | 9.8 g H2SO4��0.1NA��H2SO4���� |
| A�� | ����ǿ����HClO4��H3PO4��H2SO4 | B�� | ԭ�Ӱ뾶��С��Na��P��N | ||
| C�� | ����ǿ����NaOH��Mg��OH��2��Al��OH��3 | D�� | ������ǿ����K��Na��Li |
| A�� | OH- | B�� | Fe | C�� | H+ | D�� | SCN- |
| A�� | Ϊ������ʳ���Ӫ���ɷ֣����Դ���ʹ��ʳƷ���Ӽ� | |
| B�� | �ӵ�����ʳ���м���ⵥ�� | |
| C�� | ʯӢ�������������ά | |
| D�� | �Ӻ�ˮ����ȡ���ʶ�����ͨ����ѧ��Ӧ����ʵ�� |
 ��
�� ��
��
 +H2O
+H2O ��
�� ��
�� ����дһ�֣���
����дһ�֣��� ���̵IJ�����Ϣ��
���̵IJ�����Ϣ��