��Ŀ����
��֪��RCH2COOH
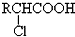 ��
��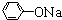 +RCl��NaCl+
+RCl��NaCl+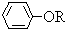
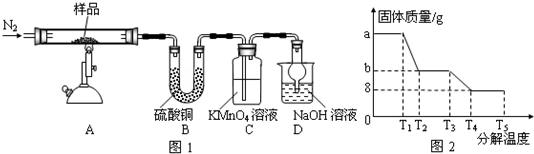
ͼ�У�B���ȴ����ᣬ��������3��̼��F�ǽ�Ѫ֬�������̴���ҩ�
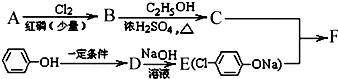
��1��AΪһԪ���ᣬ4.4g A������NaHCO3��Һ��Ӧ����1.12L CO2����״������A�ķ���ʽΪ ��
��2��B��C�ķ�Ӧ����Ϊ ��B�������ͬ���칹���� �֣�����B����
��3��D�Ľṹ��ʽ ��������ŵ�����Ϊ ��
��4��C+E��F�Ļ�ѧ��Ӧ����ʽΪ ��
| Cl2 |
| ����(����) |
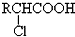 ��
��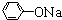 +RCl��NaCl+
+RCl��NaCl+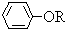
ͼ�У�B���ȴ����ᣬ��������3��̼��F�ǽ�Ѫ֬�������̴���ҩ�
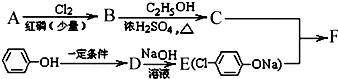
��1��AΪһԪ���ᣬ4.4g A������NaHCO3��Һ��Ӧ����1.12L CO2����״������A�ķ���ʽΪ
��2��B��C�ķ�Ӧ����Ϊ
��3��D�Ľṹ��ʽ
��4��C+E��F�Ļ�ѧ��Ӧ����ʽΪ
���㣺�л�����ƶ�
ר�⣺�л���Ļ�ѧ���ʼ��ƶ�
������AΪһԪ���ᣬ8.8gA������NaHCO3��Һ��Ӧ���� 2.24LCO2����״������������̼Ϊ0.1mol����AΪ0.1mol����A����Է�������Ϊ
=88��ȥ��1��-COOH��ʣ�����ʽ��Ϊ88-45=43����ʣ�����Ϊ-C3H7����AΪC3H7-COOH�������ʽΪC4H8O2��A����������ȡ����Ӧ����B��B���ȴ����ᣬ�Һ˴Ź��������������壬���Ƴ�BΪ ����AΪ
����AΪ ����C�Ľṹ��ʽΪ
����C�Ľṹ��ʽΪ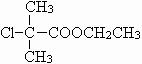 ���ɱ��ӡ�D��Eת������ϱ��ӡ�E�Ľṹ��ʽ��֪��DΪ
���ɱ��ӡ�D��Eת������ϱ��ӡ�E�Ľṹ��ʽ��֪��DΪ ���Լ�aΪ������C��F����ȡ����Ӧ����FΪ
���Լ�aΪ������C��F����ȡ����Ӧ����FΪ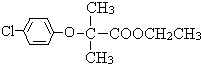 ���ݴ˽��
���ݴ˽��
| 8.8 |
| 0.1 |
 ����AΪ
����AΪ ����C�Ľṹ��ʽΪ
����C�Ľṹ��ʽΪ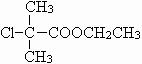 ���ɱ��ӡ�D��Eת������ϱ��ӡ�E�Ľṹ��ʽ��֪��DΪ
���ɱ��ӡ�D��Eת������ϱ��ӡ�E�Ľṹ��ʽ��֪��DΪ ���Լ�aΪ������C��F����ȡ����Ӧ����FΪ
���Լ�aΪ������C��F����ȡ����Ӧ����FΪ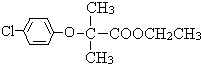 ���ݴ˽��
���ݴ˽�����
�⣺AΪһԪ���ᣬ8.8gA������NaHCO3��Һ��Ӧ���� 2.24LCO2����״������������̼Ϊ0.1mol����AΪ0.1mol����A����Է�������Ϊ
=88��ȥ��1��-COOH��ʣ�����ʽ��Ϊ88-45=43����ʣ�����Ϊ-C3H7����AΪC3H7-COOH�������ʽΪC4H8O2��A����������ȡ����Ӧ����B��B���ȴ����ᣬ�Һ˴Ź��������������壬���Ƴ�BΪ ����AΪ
����AΪ ����C�Ľṹ��ʽΪ
����C�Ľṹ��ʽΪ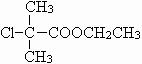 ���ɱ��ӡ�D��Eת������ϱ��ӡ�E�Ľṹ��ʽ��֪��DΪ
���ɱ��ӡ�D��Eת������ϱ��ӡ�E�Ľṹ��ʽ��֪��DΪ ���Լ�aΪ������C��F����ȡ����Ӧ����FΪ
���Լ�aΪ������C��F����ȡ����Ӧ����FΪ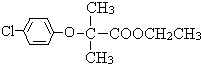 ��
��
��1��������������֪��A�ķ���ʽΪC4H8O2���ʴ�Ϊ��C4H8O2��
��2��B��C���Ȼ��봼������������Ӧ������ȡ����Ӧ��BΪ ���������ͬ���칹���У�ClCH2CH2CH2COOH��CH3CH��Cl��CH2COOH��CH3CH2CH��Cl��COOH��ClCH2CH��CH3��COOH���ʴ�Ϊ��4��
���������ͬ���칹���У�ClCH2CH2CH2COOH��CH3CH��Cl��CH2COOH��CH3CH2CH��Cl��COOH��ClCH2CH��CH3��COOH���ʴ�Ϊ��4��
��3��D�Ľṹ��ʽΪ ��������ŵ�����Ϊ���ǻ�����ԭ�ӣ��ʴ�Ϊ��
��������ŵ�����Ϊ���ǻ�����ԭ�ӣ��ʴ�Ϊ�� ���ǻ�����ԭ�ӣ�
���ǻ�����ԭ�ӣ�
��4��C+E��F�Ļ�ѧ��Ӧ����ʽΪ��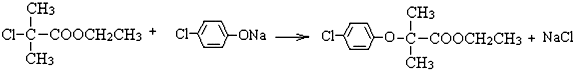 ��
��
�ʴ�Ϊ��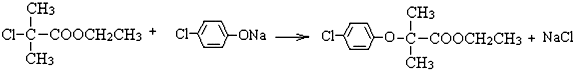 ��
��
| 8.8 |
| 0.1 |
 ����AΪ
����AΪ ����C�Ľṹ��ʽΪ
����C�Ľṹ��ʽΪ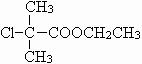 ���ɱ��ӡ�D��Eת������ϱ��ӡ�E�Ľṹ��ʽ��֪��DΪ
���ɱ��ӡ�D��Eת������ϱ��ӡ�E�Ľṹ��ʽ��֪��DΪ ���Լ�aΪ������C��F����ȡ����Ӧ����FΪ
���Լ�aΪ������C��F����ȡ����Ӧ����FΪ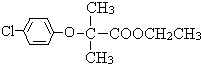 ��
����1��������������֪��A�ķ���ʽΪC4H8O2���ʴ�Ϊ��C4H8O2��
��2��B��C���Ȼ��봼������������Ӧ������ȡ����Ӧ��BΪ
 ���������ͬ���칹���У�ClCH2CH2CH2COOH��CH3CH��Cl��CH2COOH��CH3CH2CH��Cl��COOH��ClCH2CH��CH3��COOH���ʴ�Ϊ��4��
���������ͬ���칹���У�ClCH2CH2CH2COOH��CH3CH��Cl��CH2COOH��CH3CH2CH��Cl��COOH��ClCH2CH��CH3��COOH���ʴ�Ϊ��4����3��D�Ľṹ��ʽΪ
 ��������ŵ�����Ϊ���ǻ�����ԭ�ӣ��ʴ�Ϊ��
��������ŵ�����Ϊ���ǻ�����ԭ�ӣ��ʴ�Ϊ�� ���ǻ�����ԭ�ӣ�
���ǻ�����ԭ�ӣ���4��C+E��F�Ļ�ѧ��Ӧ����ʽΪ��
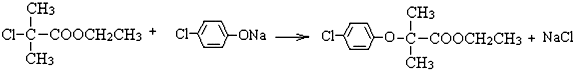 ��
���ʴ�Ϊ��
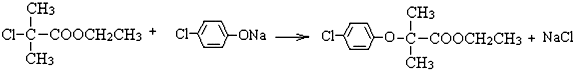 ��
��
���������⿼���л����ƶ���ϳɣ�����ȷ��A�ķ���ʽ���ۺϷ���ȷ��B�Ľṹ�ǽ���ؼ���ע���������Ϣ�����ؿ���ѧ������������������Ŀ�ѵ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
���и��������У�����˳����ȷ���ǣ�������
�������ԣ�KMnO4��MnO2��Cl2
�ڵ��ʵ��ܶȣ�Na��K��Rb
�����뾶��K+��S2-��F
�����ʵ��۵㣺Li��Na��K
���⻯��ķе㣺H2Se��H2S��H2O��
�������ԣ�KMnO4��MnO2��Cl2
�ڵ��ʵ��ܶȣ�Na��K��Rb
�����뾶��K+��S2-��F
�����ʵ��۵㣺Li��Na��K
���⻯��ķе㣺H2Se��H2S��H2O��
| A���٢� | B���ڢ� | C���٢� | D���ۢ� |
��A��B��C��D�ĸ���Ӧ���ʱ���ر������ʾ������˵����ȷ���ǣ�������
| ��Ӧ | A | B | C | D |
| ��H/kJ?mol-1 | 10.5 | 1.80 | -126 | -11.7 |
| ��S/kJ?mol-1?k-1 | 30.0 | -113.0 | 84.0 | -105.0 |
| A����ӦA���κ��¶��¾����Է����� |
| B����ӦB���κ��¶��¾������Է����� |
| C����ӦC�������¶ȸ���170��ʱ�����Է����� |
| D����ӦD���κ��¶��¾������Է����� |
����������ˮ��Һ���ܴ���������ǣ�������
| A��Ca2+��K+��Cl-��CO32- |
| B��Ba2+��Cl-��K+��SO42- |
| C��Fe3+��Cl-��K+��NO3- |
| D��Ag+��NO3-��K+��Cl- |