��Ŀ����
13��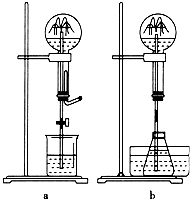 ��Ȫ��һ�ֳ�������Ȼ���������ԭ��ͨ����װ���������ѹǿ�
��Ȫ��һ�ֳ�������Ȼ���������ԭ��ͨ����װ���������ѹǿ���1��ͼa��ʾΪ��ѧ��ѧ�����õ���Ȫʵ��װ�ã�����ƿ�г����������壬��ͷ�ιܼ��ձ��зֱ�ʢ��Һ�壮��������в������γ���Ȫ����B
A��HCl��H2O B��O2��H2O
C��NH3��H2O D��CO2��NaOH��Һ
��2��ijѧ������˼��������Ȫ�������취�����������ͼb��ʾװ�ã�
����ͼb��ƿ�зֱ���������������ʣ���Ӧ����ܲ�����Ȫ����D
A��Cu��ϡ����
B��NaHCO3��NaOH��Һ
C��CaCO3��ϡ����
D��NH4HCO3��ϡ����
������ƿ���һˮ�ۣ�ƿ�м���ƾ���ˮ���м�����ˮ���ټ����������������ʣ����Ҳ��������Ȫ��ˮ���м�������ʿ�����A
A��Ũ����B��ʳ��C�������D������ͭ
���ַ���������Ȫ��ԭ����ŨH2SO4����ˮ���ȣ��¶����ߣ��ƾ��ӿ�ӷ�����ƿ����ѹ����
�۱Ƚ�ͼa��ͼb����װ�ã��Ӳ�����Ȫ��ԭ����������ͼa�Ǽ�С�������С������ͬ���ϲ���ƿ�ڵ�����ѹǿ��ͼb�������²���ƿ�ڵ�����ѹǿ��
���� ��1�������������ڽ�ͷ�ι�����Һ����ʹԲ����ƿ��ѹǿ��С�����γ���Ȫ��
��2�������ݼ���������ܵ�����ƿ����ѹ����ԭ�����ܲ�����Ȫ��֪ʶ�������
��ˮ�����¶����ߣ�ʹ�ƾ��ӷ�����ƿ����ѹ���ʹ��ƿ�е�ѹǿ���
��ͼ���ͼ������װ�ã�������ѹǿ��γ���Ȫ��
��� �⣺��1����װ�����γ���Ȫ����������ƿ�е���ѹ��С��
A��HCl������H2O��������ʹ��ƿ�е�����Ѹ�ټ�С�����γ���Ȫ����A��ѡ��
B��O2��������H2O�����Բ���ʹ��ƿ�е������С�������γ���Ȫ����Bѡ��
C��NH3������H2O��������ʹ��ƿ�е�����Ѹ�ټ�С�����γ���Ȫ����C��ѡ��
D��CO2��NaOH��Һ��Ѹ�ٷ�Ӧ���ܵ�����ƿ�е������С���������γ���Ȫ����D��ѡ��
�ʴ�Ϊ��B��
��2����ͼ2������ƿ�м���������ܵ�����ƿ����ѹ�������γ���Ȫ��
��A��Cu��ϡ�����Ӧ�����Բ��ܵ�����ƿ�е���ѹ���A����
B��NaHCO3��NaOH��Һ��Ӧ���������壬���ܵ�����ƿ�е���ѹ���B����
C��CaCO3��ϡ�������ɵ����������ˮ����ֹ��Ӧ��һ�����������ɶ�����̼�Ƚ��٣�������ƿ�е���ѹ�仯�������γ���Ȫ����C����
D��NH4HCO3��ϡ���ᷴӦ���ɴ������壬������ƿ�е���ѹ����������γ���Ȫ����D��ȷ��
�ʴ�Ϊ��D��
����ͼ2��ƿ���һˮ�ۣ�ƿ�м���ƾ���ˮ���м�����ˮ���ټ������������ʣ����Ҳ��������Ȫ���ͱ���Ҫ��ˮ���м�������ʺ���ʹ��ƿ�е��¶��������ߣ�
Ũ��������ˮ�ų���������ʹ�ƾ��������������ƾ�������ʹ��ƿ��ѹǿ������ӣ�ʳ�Ρ�����ء�����ͭ����ˮ����ʹˮ���¶����ߣ����Բ����γ���Ȫ��
�ʴ�Ϊ��A��ŨH2SO4����ˮ���ȣ��¶����ߣ��ƾ��ӿ�ӷ�����ƿ����ѹ����
��ͼ���ͼ������װ�ã�������ѹǿ��γ���Ȫ��ͼ���Ǽ�С�ϲ���ƿ������ѹǿ����ͼ���������²���ƿ������ѹǿ���ʴ�Ϊ����С������
���� ���⿼����Ȫʵ�飬Ϊ����߿��������㣬������Ȫʵ���ԭ��Ϊ���Ĺؼ���ע�ؽ��ѹǿ�ı仯���ܽ��Է���ͼ��װ������Ȫʵ��Ĺ�ϵ����ʵ�����Ƴ³��£�������ѧ�������Ŀ��飬��Ŀ�ѶȲ���

| A�� | 0.1mol/LCH3COONa��0.1mol/LHCl��Һ�������ϣ�c��Na+��=c��Cl-����c��CH3COO-����c��OH-�� | |
| B�� | 0.1mol/LNa2CO3��0.1mol/LNaHCO3��Һ�������ϣ�2c��Na+��=3c��CO32-��+3c��HCO3-��+3c��H2CO3�� | |
| C�� | Na2C2O4��Һ��HCl��Һ�������ϣ�H2C2O4�Ƕ�Ԫ���ᣩ��2c��C2O42-��+c��HC2O4-��+c��OH-��+c��Cl-��=c��Na+��+c��H+�� | |
| D�� | 0.1mol/LNH4Cl��0.1mol/L��ˮ��Һ�������ϣ�c��NH3•H2O����c��NH4+����c��Cl-����c��OH-�� |
| A�� | �к��Ȳⶨ | B�� | �к͵ζ� | ||
| C�� | ���� | D�� | ���ʵ���Ũ����Һ���� |
| A�� | a=1��b=2 | B�� | a=2��b=1 | C�� | a=2��b=2 | D�� | a=3��b=2 |
| A�� | 10.5% | B�� | 9.1% | C�� | 8% | D�� | 5% |
| A�� | ԭ�Ӱ뾶��С˳��D��C��B��A | |
| B�� | ��B��CԪ����ɵĻ�������ԼȺ������Ӽ����ֺ��й��ۼ� | |
| C�� | Ԫ��B��D��E�ֱ���A�γɵĻ������У��۷е���͵���B��A�γɵĻ����� | |
| D�� | Ԫ��D��C�γɵĻ������ڿ����г��ڷ��ò��ױ��� |
| A�� | S��Cu��Ӧ��Cu+S$\frac{\underline{\;\;��\;\;}}{\;}$CuS | |
| B�� | ������ù�����pH��С��2H2SO3+O2�T2H2SO4 | |
| C�� | �����������Һ������ʯ��ˮ��ϣ�Ca2++HSO3-+OH-�TCaSO3��+H2O | |
| D�� | ��Na2S��Na2SO3�Ļ����Һ�еμ�ϡH2SO4[n��Na2S����n��Na2SO3��=2��1]��2S2-+SO32-+6H+�T3S��+3H2O |
| A�� | ���� | B�� | ���� | C�� | ����� | D�� | ���� |
 ��1������˵����ȷ����AD������ţ�
��1������˵����ȷ����AD������ţ� ��Ni����CO�γ����������ε������Ni��CO��4��1 mol Ni��CO��4�ĺ��Ц� ������ĿΪ8NA��
��Ni����CO�γ����������ε������Ni��CO��4��1 mol Ni��CO��4�ĺ��Ц� ������ĿΪ8NA��