��Ŀ����
5��������Һ����Ũ�ȹ�ϵһ����ȷ���ǣ�������| A�� | �����£���ˮ���Ȼ�淋�pH=7�Ļ����Һ�У�c��Cl-��=c��NH4+�� | |
| B�� | ��NaHCO3��Һ�м�������NaOH���壬������HCO3-��ˮ�⣬ʹc��HCO3-������ | |
| C�� | 0.1 mol•L-1�ģ�NH4��2SO4��Һ�У�c��NH4+����c��SO42-����c��H+����c��OH-�� | |
| D�� | �����£������pH=a�Ĵ�����pH=b��NaOH��Һǡ���к�ʱ��a+b=14 |
���� A��������pH=7����Һ��c��H+��=c��OH-�������ݵ���غ��֪c��Cl-��=c��NH4+����
B������������̼��������ӷ�Ӧ����̼������Ӻ�ˮ������̼���������Ũ�ȼ�С��
C��笠����Ӳ���ˮ�⣬��Һ��ʾ���ԣ���c��H+����c��OH-��������ˮ��̶Ƚ�С����c��NH4+����c��SO42-����
D�������£������pH=a�Ĵ�����pH=b��NaOH��Һǡ���к�ʱ������ͼ�����ʵ���Ũ����ȣ�
��� �⣺A�������£���ˮ���Ȼ�淋�pH=7�Ļ����Һ��һ�����㣺c��H+��=c��OH-�������ݵ���غ�ɵã�c��Cl-��=c��NH4+������A��ȷ��
B����NaHCO3��Һ�м�������NaOH���壬������������̼��������ӷ�Ӧ��ʹc��HCO3-����С����B����
C.0.1 mol•L-1�ģ�NH4��2SO4��Һ�У�����笠����Ӳ���ˮ�⣬��Һ��ʾ���ԣ���c��H+����c��OH-��������ˮ��̶Ƚ�С����c��NH4+����c��SO42-������Һ������Ũ�ȴ�СΪ��c��NH4+����c��SO42-����c��H+����c��OH-������C��ȷ��
D�������£������pH=a�Ĵ�����pH=b��NaOH��Һǡ���к�ʱ������ͼ�����ʵ���Ũ����ȣ���c��CH3COOH��=$\frac{c��{H}^{+}��}{��}$=$\frac{1{0}^{-a}}{��}$mol/L=10 b-14mol/L��0������1����
���Ԧ�=1014-a-b��0������1���������ɵã�a+b��14����D����
��ѡAC��
���� ���⿼��������Ũ�ȴ�С�Ƚϣ���Ŀ�Ѷ��еȣ���ȷ�ε�ˮ��ԭ��������غ㡢�����غ��Ӧ�÷���Ϊ���ؼ���ע�������ж�����Ũ�ȴ�С���÷���������������ѧ�������Ӧ��������

| A�� | ��������Һ�ʹ���Ǧ��Һ����ʹ�����ʱ��� | |
| B�� | ��֬�Ǹ�֬����ĸ������������ܷ����⻯��Ӧ | |
| C�� | H2N-CH2-COOH���������ᷴӦ������������������Һ��Ӧ | |
| D�� | �ϳ���  �ĵ���֮һ��CH3-C��C-CH3 �ĵ���֮һ��CH3-C��C-CH3 |
| A�� | pH=2����Һ�У�NH4+��Na+��Cl-��Cu2+ | |
| B�� | ����KSCN�Ժ�ɫ����Һ��K+��NH4+��Cl-��CO32- | |
| C�� | ��ɫ��Һ�У�K+��CH3COO-��HCO3-��MnO4- | |
| D�� | ���д���NaClO��ˮ��Һ�У�Fe2+��Cl-��Ca2+��Na+ |
| A�� | ������ϩ������Ʒ������ʳƷ��װ | |
| B�� | H7N9�������в����ڸ��������±�ɱ���Ĺ����ǵ����ʱ��� | |
| C�� | �ߴ��ȵĹ赥�������������ά�����ά�е����� | |
| D�� | �з�ʹ�ø�Ч����������߷�Ӧ��ԭ�ϵ�ת���� |
| A�� | ��ɫֲ����й������ʱ����̫����ת��Ϊ��ѧ�ܡ����桱���� | |
| B�� | ���ʷ�����ѧ��Ӧ������һ�������������仯 | |
| C�� | �ɽ���Ӧ��NaOH+HCl�TNaCl+H2O���Ļ�ѧ��ͨ��ԭ���ת��Ϊ���� | |
| D�� | ��ѧ��Ӧʹ���Ȼ������ȣ�ȡ������������е��������ͷ�Ӧ����е������� |
 ��ͼ��ʾװ�ã���ʢ�е������Ũ�ȵ�ϡ���ᣬ����һ��ʱ�����װ����ͨ�����ӵ����ʵ�����ͬʱ������˵����ȷ���ǣ�������
��ͼ��ʾװ�ã���ʢ�е������Ũ�ȵ�ϡ���ᣬ����һ��ʱ�����װ����ͨ�����ӵ����ʵ�����ͬʱ������˵����ȷ���ǣ�������| A�� | A��������װ���е�ʯī�缫�ֱ������������� | |
| B�� | �����ĸ�ʴ�̶ȣ��ף��� | |
| C�� | ʯī�缫�Ϸ�����Ӧ�ĵ缫��Ӧʽ��ͬ | |
| D�� | ��Һ��pH����С�������� |
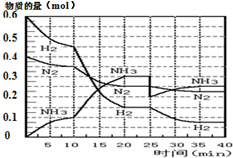 ���ĺϳ�ԭ��Ϊ��N2��g��+3H2��g��?2NH3��g������H=-92.4KJ•mol-1������500�桢20MPaʱ����N2��H2����һ���ݻ�Ϊ2L���ܱ������з�����Ӧ����Ӧ�����и����ʵ����ʵ����仯��ͼ��
���ĺϳ�ԭ��Ϊ��N2��g��+3H2��g��?2NH3��g������H=-92.4KJ•mol-1������500�桢20MPaʱ����N2��H2����һ���ݻ�Ϊ2L���ܱ������з�����Ӧ����Ӧ�����и����ʵ����ʵ����仯��ͼ���ش��������⣺
��1��10min����NH3��ʾ��ƽ����Ӧ����0.005mol/��L��min����
��2����10��20min�ڣ�NH3Ũ�ȱ仯��ԭ�������A��
A�����˴��� B����С�������
C�������¶� D������NH3���ʵ���
��3����1��ƽ���ʱ�䷶ΧΪ��20-25min����1��ƽ�⣺ƽ�ⳣ��K1=$\frac{��\frac{0.3mol}{2L}��^{2}}{��\frac{0.25mol}{2L}����{\frac{0.15mol}{2L}��}^{3}}$�������ݵı���ʽ����
��4���ڷ�Ӧ������25minʱ��
�����߷����仯��ԭ�����0.1molNH3
�ڴ�ڶ���ƽ��ʱ����ƽ���ƽ�ⳣ��K2���� K1������ڡ��������ڡ�����С�ڡ�����
��5���������¡��˹��̵������о��������ڳ��¡���ѹ�����������£�N2�ڴ�������������Fe2O3��TiO2��������ˮ�������з�Ӧ��
N2��g��+3H2O��1��?2NH3��g��+$\frac{3}{2}$O2��g������H=a kJ•mol-1
��һ���о�NH3���������¶ȵĹ�ϵ����ѹ�´ﵽƽ��ʱ��ò���ʵ���������±���
| T/K | 303 | 313 | 323 |
| NH3������/��10-6mol�� | 4.8 | 5.9 | 6.0 |
����֪��N2��g��+3H2��g��?2NH3��g����H=-92.4kJ•mol-1
2H2��g��+O2��g��=2H2O��l��=-571.6kJ•mol-1
�����µ�����ˮ��Ӧ���ɰ������������Ȼ�ѧ����ʽΪ��2N2��g��+6H2O��l��=4NH3��g��+3O2��g����H=+1536kJ•mol-1��
 ������һ����Ҫ�Ļ���ԭ�ϣ���ҵ������������̼��Ӧ�õ����ƣ���Ӧ����ʽ���£�
������һ����Ҫ�Ļ���ԭ�ϣ���ҵ������������̼��Ӧ�õ����ƣ���Ӧ����ʽ���£� H2S��
H2S�� Na2O2��
Na2O2�� ��
��