��Ŀ����
1��H2O2�dz��õ�Ư�������������ڻ���������ҽҩ����ѧ�ϳɵȷ�������Ҫ�����ã���Ϊ̽��Ӱ��H2O2�ֽ����ʵ����أ�ijʵ��С�����������ʵ�飺
ʵ��1������ͬ�������£���һ֧�Թ��м���2mL5%H2O2��1mLH2O������һ֧�Թ��м���2mL5%H2O2��1mLFeCl3��Һ���۲첢�Ƚ�ʵ������
ʵ��2��
��������ͬ��״̬��ͬ��MnO2�ֱ����ʢ��15mL5%��H2O2��Һ�Ĵ��Թ��У����ô����ǵ�ľ�����ԣ�ʵ�������£�
| MnO2 | �����Թ���� | �۲��� | ��Ӧ��������ʱ�� |
| ��ĩ״ | ���� | ���ҷ�Ӧ�������ǵ�ľ����ȼ | 3.5min |
| ��״ | �� | ��Ӧ���������Ǻ�����ľ��δ��ȼ | 30min |
��1��H2O2�ķֽⷴӦ�Ƿ��ȷ�Ӧ������Ȼ����ȣ���
��2��ʵ��1��Ŀ�����о�FeCl3��H2O2�ֽⷴӦ���ʵ�Ӱ�죬����1mLH2O�������DZ�����֧�Թ���H2O2��Ũ����ȣ�
��3��ʵ��2��ʵ���������������Ĵ�Ч��������Ӵ�����йأ�
��4��ʵ���Ҽ���Fe3+��ʵ�鷽����ȡ������FeCl3��Һ���Թ��У����뼸��KSCN��Һ����Һ��Ѫ��ɫ��
��һ�����£�H2O2��ˮ��Һ�з������ֽⷴӦ�Ĺ����У�ʵ���ò�ͬʱ��H2O2�����ʵ���Ũ�����±���
| t/min | 0 | 20 | 40 | 60 | 80 |
| C��H2O2��/mol•L-1 | 0.80 | 0.40 | 0.20 | 0.10 | 0.05 |
��2�������Ӧ���õ�H2O2��ҺΪ200mL����0��80min������O22.4g����д��������̣�
���� ��1����ʵ�������֪����Ӧ���Ȼ������ȣ�
��2���Ա�ʵ�������õ����Լ����жϳ���FeCl3��Һ��Ŀ�����о�FeCl3��H2O2�ֽⷴӦ���ʵ�Ӱ�죬��1mLH2O��Ϊ������֧�Թ���H2O2��Ũ����ȣ�
��3����ʵ�������֪�������ô�С��Ӱ�����أ�
��4��ʵ���Ҽ���ij��Һ�Ƿ���Fe3+�����õ��Լ�Ϊ���軯����Һ��
��1�����ݷ�Ӧ����V=$\frac{��c}{��t}$���㣻
��2�����ݻ�ѧ����ʽ������ϵ����õ���
��� �⣺��1�����ݴ����Թܵĸо���֪���÷�ӦΪ���ȷ�Ӧ���ʴ�Ϊ�����ȣ�
��2��һ֧�Թ��м���2mL5%H2O2��1mLH2O����һ֧�Թ��м���2mL5%H2O2��1mLFeCl3��Һ�����Եó���Ӧ��˫��ˮ��Ũ��һ�£�Ψһ�ı�������һ֧�Թ��м���1mLFeCl3��Һ���жϳ���FeCl3��Һ��Ŀ�����о�FeCl3��H2O2�ֽⷴӦ���ʵ�Ӱ�죬��1mLH2O��Ϊ������֧�Թ���H2O2��Ũ����ȣ��ʴ�Ϊ���о�FeCl3��H2O2�ֽⷴӦ���ʵ�Ӱ�죻������֧�Թ���H2O2��Ũ����ȣ�
��3����������������ͬʱ����ĩ״�������̱ȿ�״�������̷�Ӧ����ʱ��̣�˵���Ӵ�����Է�Ӧ������Ӱ�죬
�ʴ�Ϊ�������Ӵ������
��4��ʵ���Ҽ���ij��Һ�Ƿ���Fe3+�����õ��Լ�Ϊ���軯����Һ������Һ�м������軯����Һ������Һ���Ѫ��ɫ��֤����Һ�д��������ӣ�������Һ�в����������ӣ�
�ʴ�Ϊ��ȡ������FeCl3��Һ���Թ��У����뼸��KSCN��Һ����Һ��Ѫ��ɫ��
��1���÷ֽⷴӦ0��40min��ƽ����Ӧ����v��H2O2��$\frac{��c}{��t}$=$\frac{0.80mol•{L}^{-1}-0.20mol•{L}^{-1}}{40min}$=0.015mol•L-1•min-1���ʴ�Ϊ��0.015mol•L-1•min-1��
��2�������Ӧ���õ�H2O2��ҺΪ200mL��0��80min���ʸı�����ʵ���=0.2L����0.80-0.05��mol/L-=0.15mol���ɻ�ѧ����ʽ2H2O2$\frac{\underline{\;MnO_2\;}}{\;}$2H2O+O2����2H2O2��O2���õ�����O2 0.075mol��
����������Ϊ��0.075mol��32g/mol=2.4g���ʴ�Ϊ��2.4��
���� ���⿼��ͨ����ѧʵ���������Ӱ�컯ѧ��Ӧ���ʵ����⣬��Ŀ�Ѷ��еȣ�ע����������ݼ���Ӧ�����ƶϽ��ۣ������ӵļ��鷽���dz����㣬�ѶȲ���ע�ⷴӦ���ʸ��������Ӧ�ã���Ŀ�ϼ�

 ��Ԫ����ĩ��ϰ�ȷ��ϵ�д�
��Ԫ����ĩ��ϰ�ȷ��ϵ�д� ����ͬ�����Ծ�ϵ�д�
����ͬ�����Ծ�ϵ�д�| A�� | AgCl��Ag2C2O4��AgI���ߵı�����Һ��c��Ag+����С˳��Ag2C2O4��AgCl��AgI | |
| B�� | ��0.02mol•L-1AgNO3��Һ��0.02mol•L-1Na2SO4��Һ�������ϣ��г������� | |
| C�� | ��5mL����AgCl��Һ�еμ�0.5mL0.1mol•L-1KI��Һ���������� | |
| D�� | ��Ũ�Ⱦ�Ϊ0.1mol•L-1��NaCl��KI�����Һ�еμ�AgNO3��Һ�����а�ɫ�������� |
| A�� | S��O��F | B�� | S2-��Cl-��K+��Ca2+ | C�� | F-��O2-��Na+��Mg2+ | D�� | Fe��Fe2+��Fe3+ |
| A�� | ����ˮ�Ҵ��м���ŨH2SO4��������170�棬������������ͨ������KMnO4��Һ���Ϻ�ɫ��ȥ��ʹ��Һ��ɫ������һ������ϩ | |
| B�� | ��������Һ��ͨ������������̼�õ����Ӻ�̼������Һ | |
| C�� | �����������̼ԭ�Ӷ���ͬһ��ֱ���� | |
| D�� | ʵ���ҿ��õ�ʯ�뱥��ʳ��ˮ��Ӧ��ȡ��Ȳ���� |
 �����£������Ϊ10mL��pH��Ϊ12��NaX��NaY����������Һ���ֱ��ˮϡ�ͣ�pH����Һ����ı仯��ͼ��ʾ����������������ǣ�������
�����£������Ϊ10mL��pH��Ϊ12��NaX��NaY����������Һ���ֱ��ˮϡ�ͣ�pH����Һ����ı仯��ͼ��ʾ����������������ǣ�������| A�� | ��ͬ�¶��£�����ƽ�ⳣK��HX����K��HY�� | |
| B�� | b��c������Һ��ˮ�ĵ���̶���ͬ | |
| C�� | a����Һ�У�c��H+��+c��HX��=c��OH-�� | |
| D�� | b��c������Һ��Na+�����ʵ�����nb��Na+����nc��Na+�� |
| A�� | C��Mg��Li | B�� | Li��C��Mg | C�� | C��Si��Mg | D�� | C��O��Mg |
| A�� | Mһ���ǵ�VIA��Ԫ�� | B�� | M�����������ΪRO2 | ||
| C�� | M����̬�⻯��һ�����γ���� | D�� | M����̬�⻯������ˮ�����Լ��� |
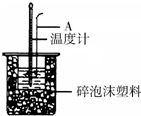 ������ͼװ�òⶨ�к��ȵ�ʵ�鲽�����£�
������ͼװ�òⶨ�к��ȵ�ʵ�鲽�����£�����ȡ50mL 0.25mol/L H2SO4��Һ����С�ձ��У������¶ȣ�
����ȡ50mL 0.55mol/L NaOH��Һ�������¶ȣ�
�۽�NaOH��Һ����С�ձ��У���Ͼ��Ⱥ�������Һ�¶ȣ���ش�
��1����ͼ��ʾ������A�������ǻ��β����������
��2��NaOH��Һ�Թ�����ԭ��ȷ�����ᱻ��ȫ�кͣ�
��3������NaOH��Һ����ȷ������B������ĸ����
A���ز������������� B��һ��Ѹ�ټ��� C�������μ���
��4��ʹ������NaOH��Һ��Ͼ��ȵ���ȷ�������û��β��������������
��5������Һ���ܶȾ�Ϊ1g•cm-3���кͺ���Һ�ı�����c=4.18J•��g•�棩-1�������ʵ������д�����к��ȵ��Ȼ�ѧ����ʽH2SO4��aq��+2NaOH��aq��Na2SO4��aq��+2H2O��l����H=-113.7kJ•mol-1
| �¶� ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶� t2/�� | �¶Ȳ�ƽ��ֵ ��t2-t1��/�� | ||
| H2SO4 | NaOH | ƽ��ֵ | |||
| 1 | 25.0 | 25.2 | 28.5 | ||
| 2 | 24.9 | 25.1 | 28.3 | ||
| 3 | 25.5 | 26.5 | 31.8 | ||
| 4 | 25.6 | 25.4 | 29.0 | ||
a��ʵ��װ�ñ��¡�����Ч����
b���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
c�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
��7����������ȷ����ȡ���Һ������¶ȣ����϶�ȡ�¶����ݣ�����¼��ֱ�������½���ȡ���ֵ��
 ���ࣻ
���ࣻ  ��������������
�������������� ���������ࣩ��
���������ࣩ��  ���ࣨ�����ᣩ��
���ࣨ�����ᣩ��