��Ŀ����
�����ڹ�ҵ�Ϸdz���Ҫ��
��1��ȡһ���������ϸС��ĩ�����ձ��У�����һ������ˮ�����Եõ������ϴ�ľ���Na2CO3?10 H2O���������ձ��еõ����﴿���ľ��壬ʵ�����Ϊ ��������ţ����ظ�ʹ�ã�
�������ᾧ �ڷ�������� ��ת����������� ����ˮϴ��2��3�� �����Ҵ�ϴ�� ��������
��2��ijС��ͬѧҪ����100mL 0.100mol?L-1 Na2CO3��Һ����ͼ��һЩ�ؼ�����Ͳ�����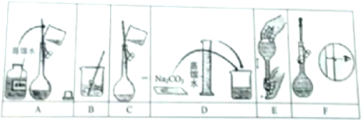
�����ƹ��̵��Ⱥ�˳��Ϊ������ĸA-F��д�� ��
����Na2CO3?10 H2O������������Һ���������Ѿ�����ʧȥ�ᾧˮ�����������Һ��Ũ�Ȼ� ���ƫ�ߡ�����ƫ�͡��������䡱��������F��Ϊ ����������ƣ���������ӿ̶��ߣ�������Һ��Ũ�Ƚ� ���ƫ�ߡ�����ƫ�͡��������䡱����
���ڲ���B֮���� �Ž�����һ��������
��3����100mL 3mol?L-1��������μ���20%��100mL����=1.06g?cm-3��Na2CO3��Һ�У���Ӧֹͣ�����״���²���������������д��������̣���
��1��ȡһ���������ϸС��ĩ�����ձ��У�����һ������ˮ�����Եõ������ϴ�ľ���Na2CO3?10 H2O���������ձ��еõ����﴿���ľ��壬ʵ�����Ϊ
�������ᾧ �ڷ�������� ��ת����������� ����ˮϴ��2��3�� �����Ҵ�ϴ�� ��������
��2��ijС��ͬѧҪ����100mL 0.100mol?L-1 Na2CO3��Һ����ͼ��һЩ�ؼ�����Ͳ�����

�����ƹ��̵��Ⱥ�˳��Ϊ������ĸA-F��д��
����Na2CO3?10 H2O������������Һ���������Ѿ�����ʧȥ�ᾧˮ�����������Һ��Ũ�Ȼ�
���ڲ���B֮����
��3����100mL 3mol?L-1��������μ���20%��100mL����=1.06g?cm-3��Na2CO3��Һ�У���Ӧֹͣ�����״���²���������������д��������̣���
���㣺����һ�����ʵ���Ũ�ȵ���Һ,��ѧ����ʽ���йؼ���
ר�⣺
��������1���������Һ���з�����������ù��˵ķ�����Ϊ�õ���������Ĺ��壬�����Ҵ�ϴ�ӣ�
��2���ٸ�������һ�����ʵ���Ũ�ȵ���Һ����ȷ����������ͼʾ�������̽�������
�ھ����Ѿ�����ʧȥ�ᾧˮ�����³���������̼���Ƶ����ʵ���ƫ�����ƽ��ƫ�ߣ�����FΪʹ�ý�ͷ�ιܶ��ݣ������Ӷ��ݣ����¼��������ˮ���ƫС��������ҺŨ��ƫ�ߣ�
��̼���ƾ������ܽ�����л�ų���������Ҫ��ȴ�����º����ת�Ƶ�����ƿ�У�
��3��������μ��뵽̼������Һ���Ⱥ���Na2CO3+HCl�TNaHCO3+NaCl��NaHCO3+HCl�TNaCl+CO2��+H2O����Ϸ�Ӧ�ķ���ʽ���㣮
��2���ٸ�������һ�����ʵ���Ũ�ȵ���Һ����ȷ����������ͼʾ�������̽�������
�ھ����Ѿ�����ʧȥ�ᾧˮ�����³���������̼���Ƶ����ʵ���ƫ�����ƽ��ƫ�ߣ�����FΪʹ�ý�ͷ�ιܶ��ݣ������Ӷ��ݣ����¼��������ˮ���ƫС��������ҺŨ��ƫ�ߣ�
��̼���ƾ������ܽ�����л�ų���������Ҫ��ȴ�����º����ת�Ƶ�����ƿ�У�
��3��������μ��뵽̼������Һ���Ⱥ���Na2CO3+HCl�TNaHCO3+NaCl��NaHCO3+HCl�TNaCl+CO2��+H2O����Ϸ�Ӧ�ķ���ʽ���㣮
���
�⣺��1��̼���ƺ���ˮ��Ӧ����̼���ƽᾧˮ�����Ӧԭ��ΪNa2CO3+10H2O=Na2CO3?10 H2O���������Һ���з�����������ù��˵ķ�����Ϊ�õ���������Ĺ��壬�����Ҵ�ϴ�ӣ���������������У��ʴ�Ϊ���ۢݢڣ�
��2��������һ�����ʵ���Ũ�ȵ���Һ����Ϊ�����㡢�������ܽ⡢��ȴ��ת�ơ�ϴ�ӡ����ݡ�ҡ�ȵȣ�������ȷ��˳��Ϊ��DBCAFE��
�ʴ�Ϊ��DBCAFE��
����Na2CO3?10H2O��������Һ���������Ѿ�����ʧȥ�ᾧˮ��������̼���ƾ����к��е�̼���Ƶ����ʵ���ƫ�����������Һ��Ũ�Ȼ�ƫ�ߣ�����FΪ���ݣ�����ʱ�۾�Ӧ��ƽ������ƿ�̶��ߣ������Ӱ�����ƽ���������Ӷ��ݣ����¼��������ˮ���ƫС�����Ƶ���ҺŨ��ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ����ݣ�ƫ�ߣ�
��̼���ƾ������ܽ�ʱ��ų��������¶Ȼ�Ӱ����Һ����������Ա������Һ��ȴ�����º���ת�Ƶ�����ƿ�У������Ӱ�����ƽ�����ʴ�Ϊ����ȴ�����£�
��3��n��HCl��=3mol/L��0.1L=0.3mol��n��Na2CO3��=
=0.2mol��
���ȷ�����Na2CO3+HCl�TNaHCO3+NaCl��
0.2mol 0.2mol 0.2mol
������NaHCO3+HCl�TNaCl+CO2��+H2O
0.1mol 0.1mol 0.1mol
������0.1mol CO2�����Ϊ0.1mol��22.4L/mol=2.24L��
�𣺱�״���²�����������Ϊ2.24L��
��2��������һ�����ʵ���Ũ�ȵ���Һ����Ϊ�����㡢�������ܽ⡢��ȴ��ת�ơ�ϴ�ӡ����ݡ�ҡ�ȵȣ�������ȷ��˳��Ϊ��DBCAFE��
�ʴ�Ϊ��DBCAFE��
����Na2CO3?10H2O��������Һ���������Ѿ�����ʧȥ�ᾧˮ��������̼���ƾ����к��е�̼���Ƶ����ʵ���ƫ�����������Һ��Ũ�Ȼ�ƫ�ߣ�����FΪ���ݣ�����ʱ�۾�Ӧ��ƽ������ƿ�̶��ߣ������Ӱ�����ƽ���������Ӷ��ݣ����¼��������ˮ���ƫС�����Ƶ���ҺŨ��ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ����ݣ�ƫ�ߣ�
��̼���ƾ������ܽ�ʱ��ų��������¶Ȼ�Ӱ����Һ����������Ա������Һ��ȴ�����º���ת�Ƶ�����ƿ�У������Ӱ�����ƽ�����ʴ�Ϊ����ȴ�����£�
��3��n��HCl��=3mol/L��0.1L=0.3mol��n��Na2CO3��=
| 100mL��1.06g/mL��20% |
| 106g/mol |
���ȷ�����Na2CO3+HCl�TNaHCO3+NaCl��
0.2mol 0.2mol 0.2mol
������NaHCO3+HCl�TNaCl+CO2��+H2O
0.1mol 0.1mol 0.1mol
������0.1mol CO2�����Ϊ0.1mol��22.4L/mol=2.24L��
�𣺱�״���²�����������Ϊ2.24L��
���������⿼���Ƶ���Ҫ����������ڴ�������ʼ���ѧ�����֪ʶ������һ�����ʵ���Ũ�ȵ���Һ�ķ���������������Ŀ�ѶȲ���ע��������ȷ�����Ʒ�����������������Ϊ�Ѷȣ�ע����ȷ�������ķ��������ɣ�

��ϰ��ϵ�д�
 ��ӡ�Ļ���ʱ����ϵ�д�
��ӡ�Ļ���ʱ����ϵ�д� ��ѧ�����ϵ�д�
��ѧ�����ϵ�д�
�����Ŀ
���з�Ӧ�����ӷ���ʽ��д��ȷ���ǣ�������
| A���Ȼ�����Һ�м��������ˮ��Al3++4NH3?H2O=AlO2-+4NH4++2H2O |
| B��NaHCO3��Һ������Ba��OH��2��Һ��ϣ�HCO3-+OH-+Ba2+�TH2O+BaCO3�� |
| C��NaHCO3��Һ����Ba��OH��2��Һ��ϣ�2HCO3-+2OH-+Ba2+�TBaCO3��+2H2O+CO32- |
| D��NaAlO2��Һ��ͨ������CO2��2A1O2-+CO2+3H2O�T2Al��OH��3��+CO32- |
�����й����ʱ����˵������ȷ���ǣ�������
| A���Ʊ�����ú���� |
| B����ˮ�ɳ��ڱ�������ɫƿ�� |
| C����������Ӧ�ܷⱣ�� |
| D��Ư�۲�Ӧ�����ڿ����� |


 ij��ѧ��ȤС���ͬѧΪ̽����������Ļ�ѧ���ʣ��������ͼ��ʾ��װ�ã�
ij��ѧ��ȤС���ͬѧΪ̽����������Ļ�ѧ���ʣ��������ͼ��ʾ��װ�ã�