��Ŀ����
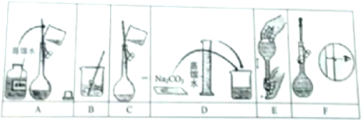
 ij��ѧ��ȤС���ͬѧΪ̽����������Ļ�ѧ���ʣ��������ͼ��ʾ��װ�ã�
ij��ѧ��ȤС���ͬѧΪ̽����������Ļ�ѧ���ʣ��������ͼ��ʾ��װ�ã���ش��������⣮
��1��ͭ��Ũ���ᷴӦ�Ļ�ѧ����ʽΪ
��2��Bƿ��ʢ��Ʒ����Һ���۲쵽Ʒ����Һ��ɫ��������ΪSO2����
A�������ԡ�������������B����ԭ�ԡ�������C��Ư����
��3��Dƿ��ʢ��NaOH��Һ��������
��4����ַ�Ӧ��С��ͬѧ����ͭ�����ᶼ��ʣ�࣮����ʹʣ���ͭƬ�ܽ⣬���ټ���
A��HNO3����������B��NaNO3��������������C��NaHCO3����������D��Na2CO3
��5��Ϊ��һ������SO2����Ⱦ�����Ϊ�����ҹ�����̽����һ����������CO��ԭSO2�õ�������ķ�������ȥSO2��д���÷�Ӧ�Ļ�ѧ����ʽ��
���㣺̽������������ˮ��Ʒ����Һ�ķ�Ӧ,��������Ļ�ѧ����
ר�⣺ʵ�������
��������1�����������£�Cu��Ũ���ᷢ��������ԭ��Ӧ��������ͭ�����������ˮ��
��2�����������ܺ���ɫ���ʷ�Ӧ������ɫ���ʶ�����Ư���ԣ��ܱ�ǿ���������������ֻ�ԭ�ԣ�
��3�����������ж�������ֱ���ſգ��Ҷ���������������������ܺ�ǿ����Һ��Ӧ��
��4�������£�Cu��ϡ�����Ӧ�����ܺ����ᷴӦ��Ҫʹʣ���Cu�ܽ⣬Ӧ�ü��뺬����������ӵ����ʣ�
��5��һ�������£�CO��ԭSO2�õ��������CO2��
��2�����������ܺ���ɫ���ʷ�Ӧ������ɫ���ʶ�����Ư���ԣ��ܱ�ǿ���������������ֻ�ԭ�ԣ�
��3�����������ж�������ֱ���ſգ��Ҷ���������������������ܺ�ǿ����Һ��Ӧ��
��4�������£�Cu��ϡ�����Ӧ�����ܺ����ᷴӦ��Ҫʹʣ���Cu�ܽ⣬Ӧ�ü��뺬����������ӵ����ʣ�
��5��һ�������£�CO��ԭSO2�õ��������CO2��
���
�⣺��1�����������£�Cu��Ũ���ᷢ��������ԭ��Ӧ��������ͭ�����������ˮ����Ӧ����ʽΪCu+2H2SO4��Ũ��
CuSO4+SO2��+2H2O���ʴ�Ϊ��Cu+2H2SO4��Ũ��
CuSO4+SO2��+2H2O��
��2�����������ܺ���ɫ���ʷ�Ӧ������ɫ���ʶ�����Ư���ԣ�����������ʹƷ����Һ��ɫ˵������Ư���ԣ���������ǿ�����ԣ��ܽ��������������������ᡢ��������ԭ�������ᣬ����������ɫ����ʵ��˵������������л�ԭ�ԣ��ʴ�Ϊ��C��B��
��3�����������ж�������ֱ���ſգ��Ҷ���������������������ܺ�ǿ��NaOH��Һ��Ӧ�����������ƺ�ˮ�����ӷ���ʽΪSO2+2OH-=SO32-+H2O������NaOH��Һ������������β�����ʴ�Ϊ������β����SO2+2OH-=SO32-+H2O��
��4�������£�Cu��ϡ�����Ӧ�����ܺ����ᷴӦ��ͭ��Ũ���ᷴӦ�����Һ�����ԣ�������������������Ӿ���ǿ�����ԣ�����Ҫʹʣ���Cu�ܽ⣬Ӧ�ü��뺬����������ӵ����ʼ��ɣ���ѡAB��
��5��һ�������£�CO��ԭSO2�õ��������CO2����Ӧ����ʽΪ2CO+SO2
S+2CO2���ʴ�Ϊ��2CO+SO2
S+2CO2��
| ||
| ||
��2�����������ܺ���ɫ���ʷ�Ӧ������ɫ���ʶ�����Ư���ԣ�����������ʹƷ����Һ��ɫ˵������Ư���ԣ���������ǿ�����ԣ��ܽ��������������������ᡢ��������ԭ�������ᣬ����������ɫ����ʵ��˵������������л�ԭ�ԣ��ʴ�Ϊ��C��B��
��3�����������ж�������ֱ���ſգ��Ҷ���������������������ܺ�ǿ��NaOH��Һ��Ӧ�����������ƺ�ˮ�����ӷ���ʽΪSO2+2OH-=SO32-+H2O������NaOH��Һ������������β�����ʴ�Ϊ������β����SO2+2OH-=SO32-+H2O��
��4�������£�Cu��ϡ�����Ӧ�����ܺ����ᷴӦ��ͭ��Ũ���ᷴӦ�����Һ�����ԣ�������������������Ӿ���ǿ�����ԣ�����Ҫʹʣ���Cu�ܽ⣬Ӧ�ü��뺬����������ӵ����ʼ��ɣ���ѡAB��
��5��һ�������£�CO��ԭSO2�õ��������CO2����Ӧ����ʽΪ2CO+SO2
| ||
| ||
���������⿼�������������ʣ����ؿ���ʵ�������������������ȷ�����������Ư���ԡ������ԡ���ԭ�ԣ�֪����������Ư���Ժʹ�����Ư����ԭ����������Ŀ�ѶȲ���

��ϰ��ϵ�д�
 �������ϵ�д�
�������ϵ�д�
�����Ŀ
 ��ͼ��C%��ʾij��Ӧ������ϵ�еİٷֺ�����v��ʾ��Ӧ���ʣ�P��ʾѹǿ��t��ʾ��Ӧʱ�䣮��ͼ��A��Ϊ�¶�һ��ʱ��ѹǿ�뷴Ӧ���ʵĹ�ϵ���ߣ���ͼ��B��Ϊѹǿһ��ʱ���ڲ�ͬʱ��C%���¶ȵĹ�ϵ���ߣ�ͬʱ������������ͼ��ķ�Ӧ�ǣ�������
��ͼ��C%��ʾij��Ӧ������ϵ�еİٷֺ�����v��ʾ��Ӧ���ʣ�P��ʾѹǿ��t��ʾ��Ӧʱ�䣮��ͼ��A��Ϊ�¶�һ��ʱ��ѹǿ�뷴Ӧ���ʵĹ�ϵ���ߣ���ͼ��B��Ϊѹǿһ��ʱ���ڲ�ͬʱ��C%���¶ȵĹ�ϵ���ߣ�ͬʱ������������ͼ��ķ�Ӧ�ǣ�������| A��4NH2��g��+5O2��g��?4NO��g��+6H2O��g����H=-808.7Kj/mol |
| B��N2O3��g��?NO2��g��+NO��g����H=+41.8Kj/mol |
| C��3NO2��g��+H2O��l��?2HNO2��l��+NO��g����H=-261.3Kj/mol |
| D��CO2��g��+C��s��?2CO��g����H=+171.4Kj/mol |
���ж������������ȷ���ǣ�������
| A����Ũ�������ǿ�����ԣ��ʲ����������������� |
| B��Ũ������Ũ��ˮ����ʱ������������ |
| C��Ũ������ǿ�����ԣ�ϡ������������� |
| D��Ũ������и�ʴ�ԣ�ȡ��ʱҪС�� |
���ڿ��淴Ӧ��FeO��s��+CO��g��?Fe��s��+CO2��g����һ���¶�����ƽ�ⳣ��K�����������仯�У���ʹK�����仯���ǣ�������
| A������FeO��s������� |
| B��������ϵѹǿ |
| C��������ϵ�¶� |
| D��ʹ���ʺϵĴ��� |
�����£���H2O2��Һ�μ�����FeSO4��Һ���ɷ�������������Ӧ������˵����ȷ���� ��������
2Fe2++H2O2+2H+=2Fe3++2H2O
2Fe3++H2O2=2Fe2++O2��+2H+��
2Fe2++H2O2+2H+=2Fe3++2H2O
2Fe3++H2O2=2Fe2++O2��+2H+��
| A��H2O2�������Ա�Fe3+ǿ���仹ԭ�Ա�Fe2+�� |
| B����H2O2�ֽ�����У���Һ��H+Ũ�����½� |
| C����H2O2�ֽ�����У�Fe2+��Fe3+���������ֲ��� |
| D��H2O2����������������Ҫ��������Fe2+����߲��� |