��Ŀ����
2��������H��������·�ߺϳɣ�
��֪��R-CH=CH2$\underset{\stackrel{��i��{B}_{2}{H}_{6}}{��}}{��ii��{H}_{2}{O}_{2}/O{H}^{-}}$RCH2CH2OH
�ش��������⣺
��1��11.2L����״��������A�������г��ȼ�տ�������88g CO2��45g H2O����A���ӽṹ����3��������A�Ľṹ��ʽΪCH3CH��CH3��CH3��
��2��B��C��Ϊһ�ȴ�����D�����ƣ�ϵͳ������Ϊ2-����ϩ��
��3���ڴ���������1mol F��2mol H2��Ӧ������3-����-1-������F�Ľṹ��ʽ��
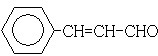 ��
����4��G�й�����������̼̼˫�����Ȼ��������������G�ķ�����ͬ���칹������4��
��5����Ӧ�ڵĻ�ѧ����ʽΪ
 ��
��
���� 88gCO2�����ʵ���Ϊ��$\frac{88g}{44g/mol}$=2mol��45gH2O�����ʵ���Ϊ��$\frac{45g}{18g/mol}$=2.5mol�������11.2L��AΪ�����ʵ���Ϊ��$\frac{11.2L}{22.4L/mol}$=0.5mol��������A�к�̼ԭ��Ϊ4��Hԭ����Ϊ10����A��ѧʽΪC4H10��A�к���3��������AΪCH3CH��CH3��CH3��A��Cl2���շ���һ��ȡ��ʱ�����ֲ��2-��-1-�ȱ����2-��-2-�ȱ��飬��NaOH����Һ�����·�����ȥ��Ӧ����DΪCH2=C��CH3��2��D������Ϣ�еķ�Ӧ����EΪ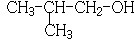 ��F������Cu��OH��2��Ӧ������ȩ����1mol F��2mol H2��Ӧ����3-����-1-����������̼̼˫������F�Ľṹ��ʽΪ
��F������Cu��OH��2��Ӧ������ȩ����1mol F��2mol H2��Ӧ����3-����-1-����������̼̼˫������F�Ľṹ��ʽΪ 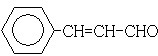 ��F��ȩ������Ϊ�Ȼ�����GΪ
��F��ȩ������Ϊ�Ȼ�����GΪ ��D��E����������Ӧ����HΪ
��D��E����������Ӧ����HΪ ���ݴ˽��
���ݴ˽��
��� �⣺88gCO2�����ʵ���Ϊ��$\frac{88g}{44g/mol}$=2mol��45gH2O�����ʵ���Ϊ��$\frac{45g}{18g/mol}$=2.5mol�������11.2L��AΪ�����ʵ���Ϊ��$\frac{11.2L}{22.4L/mol}$=0.5mol��������A�к�̼ԭ��Ϊ4��Hԭ����Ϊ10����A��ѧʽΪC4H10��A�к���3��������AΪCH3CH��CH3��CH3��A��Cl2���շ���һ��ȡ��ʱ�����ֲ��2-��-1-�ȱ����2-��-2-�ȱ��飬��NaOH����Һ�����·�����ȥ��Ӧ����DΪCH2=C��CH3��2��D������Ϣ�еķ�Ӧ����EΪ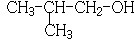 ��F������Cu��OH��2��Ӧ������ȩ����1mol F��2mol H2��Ӧ����3-����-1-����������̼̼˫������F�Ľṹ��ʽΪ
��F������Cu��OH��2��Ӧ������ȩ����1mol F��2mol H2��Ӧ����3-����-1-����������̼̼˫������F�Ľṹ��ʽΪ 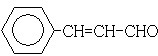 ��F��ȩ������Ϊ�Ȼ�����GΪ
��F��ȩ������Ϊ�Ȼ�����GΪ ��D��E����������Ӧ����HΪ
��D��E����������Ӧ����HΪ ��
��
��1�����ݷ�����֪��A�Ľṹ��ʽΪ��CH3CH��CH3��CH3���ʴ�Ϊ��CH3CH��CH3��CH3��
��2��D�Ľṹ��ʽΪ��CH2=C��CH3��2�����л�������Ϊ��2-����ϩ���ʴ�Ϊ��2-����ϩ��
��3��������������֪��F�Ľṹ��ʽΪ 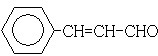 ��
��
�ʴ�Ϊ�� ��
��
��4��GΪ �����й�������̼̼˫�����Ȼ��������������G�ķ�����ͬ���칹�壬���Խ�����������Ӧ��λ�ñ任���ó��䷼�����ͬ���칹��Ϊ
�����й�������̼̼˫�����Ȼ��������������G�ķ�����ͬ���칹�壬���Խ�����������Ӧ��λ�ñ任���ó��䷼�����ͬ���칹��Ϊ ��
�� ��
�� ��
�� ������4�֣�
������4�֣�
�ʴ�Ϊ��̼̼˫�����Ȼ���4��
��5����Ӧ�ڵĻ�ѧ����ʽΪ��
�ʴ�Ϊ�� ��
��
���� �����л�����ƶ���ϳɣ��漰����ʽ��ȷ�����л�����������ѧ����ʽ����д��ͬ���칹����жϵȣ�ע����ݷ�Ӧ������������Ϣ�����ƶϣ���Ҫѧ���������չ����ŵ�������ת�����Ѷ��еȣ�

 25��ʱ����10mL 0.2mol•L-1 NaCN��Һ�м���0.2mol•L-1���ᣬ��ҺpH��������������仯�����ͼ��ʾ����֪��Ka��HCN��=6.4��10-10����������������ǣ�������
25��ʱ����10mL 0.2mol•L-1 NaCN��Һ�м���0.2mol•L-1���ᣬ��ҺpH��������������仯�����ͼ��ʾ����֪��Ka��HCN��=6.4��10-10����������������ǣ�������| A�� | a��ʱ��CN-����Ũ�ȴ��������� | B�� | b��ʱ��c��HCN����c��CN-�� | ||
| C�� | c��ʱ��c��Na+��=c��Cl-��+c��CN-�� | D�� | d��ʱ����Һ��c��H+����8��10-5mol•L-1 |
| A�� | ������ | B�� | ������ | C�� | �м��� | D�� | ˮ�����õ� |


 ��18mol/L��Ũ��������150.00mL 1.00mol/L���
��18mol/L��Ũ��������150.00mL 1.00mol/L��� ��
�� ��
�� ����B����ˮ�Ĺ����д��ڵĿ��淴ӦʽΪNH3+H2O
����B����ˮ�Ĺ����д��ڵĿ��淴ӦʽΪNH3+H2O  NH3•H2O
NH3•H2O  NH4++OH-��
NH4++OH-�� �����������ˮ����Һ�ʼ�ᡢ������ԣ���д������ˮ��Ӧ�Ļ�ѧ����ʽNH5+H2O=NH3•H2O+H2����
�����������ˮ����Һ�ʼ�ᡢ������ԣ���д������ˮ��Ӧ�Ļ�ѧ����ʽNH5+H2O=NH3•H2O+H2����