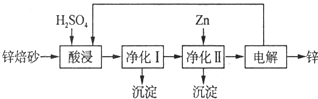
��Ŀ����
3�� ijʵ��С������ͼ��ʾ��װ���Ʊ�һ�����ױ������������ױ��Ͷ������ױ�����
ijʵ��С������ͼ��ʾ��װ���Ʊ�һ�����ױ������������ױ��Ͷ������ױ�������Ӧԭ����

ʵ�鲽�裺������Ũ������Ũ���ᣨ�������1��3���Ļ����Һ�������ᣩ40mL��
��������ƿ�м���15mL��13g���ױ���
�۰�ͼ��ʾװ��ҩƷ����װ������������
��������ƿ�м�����ᣬ�����Ͻ��裨��������������ȥ����
�ݿ����¶�ԼΪ50�棬��Ӧ��Լ10min������ƿ���д�������ɫ��״Һ����֣�
�����һ�����ױ���
ʵ���п����õ������ݣ�
| �ܶ�g•cm-3 | �е�/�� | �ܽ��� | |
| �ױ� | 0.866 | 110.6 | ������ˮ�������������ױ� |
| ��Ʒ1 | 1.286 | 237.7 | ������ˮ��������Һ���� |
| ��Ʒ2 | 1.162 | 222 | ������ˮ��������Һ���� |
��1������40mL����IJ����ǽ�30mLŨ����ע���ձ��У������ձ��ڱ�ע��10mLŨ���ᣬ�ӱ߽��裻
��2����ʵ����Ũ����������Ǵ�������ˮ����
��3��װ��������Aʹ��ǰ����ϴ�ɾ�����©��
��4�������Ʒ�ķ������£�

����2����IJ�����������6�֣�
��5�������յõ���Ʒ1�Ͳ�Ʒ2��������Ϊ17.42g����һ�����ױ����ܲ�����89.99%��������λС����
���� �Ʊ�һ�����ױ��������ƻ����Һ�������ᣩ��20mL����ӦҺ��е�ϵͣ�����ʱ���������У�����������ƿ�м����ʯ��Ȼ�����10ml�ױ����ӷ��е�Ϊ110.6�棩����������ƿ�м�����ᣬˮԡ��ˮ�ķе�100�棩Ŀ���ܾ��ȼ��ȣ��ұ��ڿ��Ʒ�Ӧ���ʣ��¶�ԼΪ50�棬��Ӧ��Լ10min������ƿ���д�������ɫ��״Һ����֣�����һ���������ﶼΪ�л������ֻ���������Ƿе�IJ�ͬ��������ķ������룬���ᴿ���յõ�������һ�����ױ���
��1�������Һ�����ƿɲ���Ũ�����ϡ�ͣ�
��2���Ʊ�һ�����ױ���Ũ�������������÷�ӦΪ���淴Ӧ��Ũ������ˮ����ƽ��������һ�����ױ������ƶ���
��3������A�Ƿ�Һ©����ʹ��ǰ�������Ƿ�©Һ��
��4����Ϊ��������һ����������ֻ���������Ƿе�IJ�ͬ����˲�������ķ�����ʹ�õ����ṩ�����еķֱ��ǣ��ƾ��ơ��¶ȼơ������ܣ�
��5�����ʵļ��㷽��������ʵ�ʲ����������۲�������駷���ʽ��һĦ���ļױ����Եõ�һĦ���Ķ������ױ���һĦ�����������ױ������������Ļ�Ӧ���ǣ�92g�ļױ����Եõ�137g�Ķ���������137g��������������ô13g�ļױ����ܵõ���һ���������������ǣ�$\frac{13��137}{92}$=19.36g���Դ˼���һ�������IJ�����
��� �⣺��1�����ƻ����Һʱ������������Ũ�����ϡ��һ�����ֱ���ȡ10 mL��30mL��Ũ�����Ũ���ᣬ��Ũ���ᵹ���ձ��У�Ũ���������ձ��ڱڻ���ע�룬�����Ͻ��裬���ƻ����Һ�������ᣩ��40mL��
�ʴ�Ϊ����30mLŨ����ע���ձ��У������ձ��ڱ�ע��10mLŨ���ᣬ�ӱ߽��裻
��2���Ʊ�һ�����ױ���Ũ�������������÷�ӦΪ���淴Ӧ��Ũ������ˮ����ƽ��������һ�����ױ������ƶ�����Ũ���������Ϊ��������ˮ����
�ʴ�Ϊ����������ˮ����
��3������A�Ƿ�Һ©����ʹ��ǰ�������Ƿ�©Һ��
�ʴ�Ϊ ��©��
��4����Ϊ��������һ����������ֻ���������Ƿе�IJ�ͬ����˲�������ķ�����ʹ�õ����ṩ�����еķֱ��ǣ�������ƿ���ƾ��ơ��¶ȼơ������ܡ���ƿ��β�ӹܣ�
�ʴ�Ϊ��6��
��5�����ʵļ��㷽��������ʵ�ʲ����������۲�������駷���ʽ��һĦ���ļױ����Եõ�һĦ���Ķ������ױ���һĦ�����������ױ������������Ļ�Ӧ���ǣ�92g�ļױ����Եõ�137g�Ķ���������137g��������������ô13g�ļױ����ܵõ���һ���������������ǣ�$\frac{13��137}{92}$=19.36g��һ�������IJ���Ϊ$\frac{17.42}{19.36}$��100%=89.99%��
�ʴ�Ϊ��89.99%��
���� ������Ҫ����һ�����ױ����Ʊ����漰ʵ�����̡����ʵļ����֪ʶ���ѶȲ�����Ϥ���̵�������Ŀ���ǽ���Ĺؼ���

 ��ʦ������Ԫ��ĩ���100��ϵ�д�
��ʦ������Ԫ��ĩ���100��ϵ�д� ��У������Ԫͬ��ѵ��������ϵ�д�
��У������Ԫͬ��ѵ��������ϵ�д�| A�� | ��ɫ������Һ�У�Na+��SO42-��MnO4-��NO3- | |
| B�� | ʹ��ɫ��̪��Һ�ʺ�ɫ����Һ�У�Na+��Cu2+��SO42-��Cl- | |
| C�� | pH=1����Һ�У�K+��ClO-��S2-��Cl- | |
| D�� | �����Ե���Һ�У�Na+��K+��HCO3-��NO3- |
��1��ȷ����8.2g�������������������ʵ���Ʒ�����500mL������Һ������ʱ����Ʒ�ɷ���A��������ĸ��������
A��С�ձ��С�������B���ྻֽƬ�ϡ�������C��������
��2���ζ�ʱ����0.2000mol•L-1���������ζ�������Һ������ѡ��B��������ĸ����ָʾ����
A�����ȡ���B��ʯ�� C����̪����D������
��3���ζ������У��۾�Ӧע���۾�ע����ƿ����Һ��ɫ�仯��������̨�ϵ�һ�Ű�ֽ����Ŀ���DZ��ڹ۲���Һ��ɫ�仯��
��4�������±����ݣ����㱻���ռ���Һ�����ʵ���Ũ����0.4000mol•L-1���ռ���Ʒ�Ĵ�����97.56%��
| �ζ����� | ������Һ �����mL�� | ������� | |
| �ζ�ǰ�Ŀ̶� ��mL�� | �ζ���Ŀ̶� ��mL�� | ||
| ��һ�� | 10.00 | 0.40 | 20.50 |
| �ڶ��� | 10.00 | 4.10 | 24.00 |
�ٹ۲���ʽ�ζ���Һ��ʱ����ʼ���ӣ��ζ��յ�ƽ�ӣ���ζ����ƫ�ߣ�
��������ƿ�ô���Һ��ϴ��Ȼ���ټ���10.00mL����Һ����ζ����ƫ�ߣ�
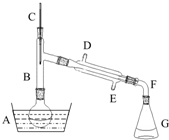 ��������һ����Ҫ���л�����ԭ�ϣ��Ʊ��������ԭ����95%�Ҵ���80%���ᣨ������ˮϡ��Ũ���ᣩ����ϸ���廯�Ʒ�ĩ�ͼ������Ƭ���÷�Ӧ��ԭ�����£�NaBr+H2SO4=NaHSO4+HBr
��������һ����Ҫ���л�����ԭ�ϣ��Ʊ��������ԭ����95%�Ҵ���80%���ᣨ������ˮϡ��Ũ���ᣩ����ϸ���廯�Ʒ�ĩ�ͼ������Ƭ���÷�Ӧ��ԭ�����£�NaBr+H2SO4=NaHSO4+HBrCH3CH2OH+HBr $\stackrel{����}{��}$CH3CH2Br+H2O
ij����С������ʵ�����Ʊ��������װ����ͼ���������±���
| ���� ���� | �Ҵ� | ������ | 1��2-�������� | ���� | Ũ���� |
| �ܶ�/g•cm-3 | 0.79 | 1.46 | 2.2 | 0.71 | 1.84 |
| �۵㣨�棩 | -130 | -119 | 9 | -116 | 10 |
| �е㣨�棩 | 78.5 | 38.4 | 132 | 34.6 | 338 |
| ��ˮ�е��ܽ�ȣ�g�� | ���� | 0.914 | 1 | 7.5 | ���� |
��1������ҩƷ֮ǰ�����IJ����Ǽ��װ�õ������ԣ�
��2������B�������dz���ʹ���������������һ��Ŀ���������������¶ȼƵ��¶�Ӧ������38.4�桫78.5�棻��ȴˮ������ӦΪE��D�����D��E������E��D������
��3����Ӧʱ�п�������SO2��һ�ֺ���ɫ���壬��ѡ��NaOH��Һ�ֱ��ȥ��Щ���壬�йص����ӷ���ʽ��SO2+2OH-=SO32-+H2O��Br2+2OH-=Br-+BrO-+H2O��
��4��ʵ���в���80%���ᣬ��������98%Ũ���ᣬһ������Ϊ�˼��ٸ���Ӧ�������HBr������������һ������Ϊ�˷�ֹ�廯������Ļӷ���
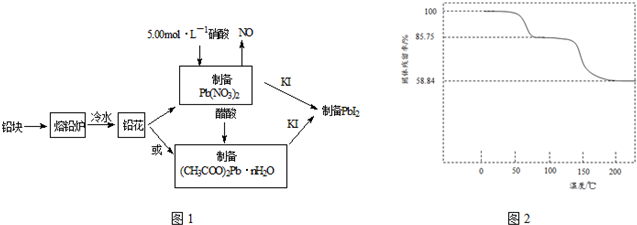
��1����Ǧ���Ƴ�Ǧ����Ŀ������������ĽӴ�������ӿ��ܽⷴӦ���ʣ�
��2��31.05gǦ����5.00mol•L-1�������ܽ⣬����������5.00mol•L-1����80mL��
��3��ȡһ��������CH3COO��2Pb•nH2O��Ʒ��N2�����м��ȣ������Ʒ��������ʣ���$\frac{������Ʒ��ʣ������}{������Ʒ����ʼ����}$��100%�����¶ȵı仯��ͼ2��ʾ����֪����Ʒ��75��ʱ����ȫʧȥ�ᾧˮ����
�٣�CH3COO��2Pb•nH2O�нᾧˮ��Ŀn=3������������
��100��200���ֽ����ΪǦ���������һ���л������л���ΪC4H6O3��д����ʽ����
��4����ȡһ��������PbI2���壬������ˮ���Ƴ�����ʱ�ı�����Һ��ȷ��ȡ25.00mL PbI2������Һ�ִμ��������ӽ�����֬RH�У�������2RH��s��+Pb2+��aq��=R2Pb��s��+2H+��aq��������ƿ��������Һ�����������ˮ��ϴ��֬������Һ�����ԣ���ϴ��Һ�ϲ�����ƿ�У�����2��3�η�̪��Һ����0.002500mol•L-1NaOH��Һ�ζ������ζ��յ�ʱ��ȥ�������Ʊ���Һ20.00mL��������ʱPbI2 ��KspΪ4.000��10-9��
��5��̽��Ũ�ȶԻǻ�Ǧ�����ܽ�ƽ���Ӱ�죮
�û�ѧС��������ṩ�Լ��������ʵ�飬��˵��Ũ�ȶԳ����ܽ�ƽ���Ӱ�죮
�ṩ�Լ���NaI������Һ��NaCl������Һ��FeCl3 ������Һ��PbI2������Һ��PbI2����Һ��
��Ϣ��ʾ��Pb2+��Cl-���γɽ��ȶ���PbCl42-�����ӣ�
����д�±��Ŀհ״���
| ʵ������ | ʵ�鷽�� | ʵ������ԭ����� |
| �ٻ�����Ũ�������ƽ���Ӱ�� | ȡPbI2������Һ������һ֧�Թ��У��ٵ��뼸��NaI������Һ | ������Һ��c��I-������ʹQ������PbI2��Ksp |
| ��Ǧ����Ũ�ȼ�С��ƽ���Ӱ�� | ȡPbI2����Һ������һ֧�Թ��У��ټ�������NaCl������Һ | ����ɫ������ʧ ԭ���γ�PbCl42-��������Һ��c��Pb2+����С��ʹQcС��PbI2��Ksp |
| ��Ǧ���Ӻ͵�����Ũ�ȶ���С��ƽ���Ӱ�� | ��PbI2����Һ�е��뼸��FeCl3 ������Һ | ����ɫ������ʧ д����Ӧ�����ӷ���ʽ�� PbI2+2Fe3++4Cl-=PbCl42-+2Fe2++I2 |

��1������O2��H2O��g���Ĵ��ڶԸ�ʵ���в���Ӱ�죬ʵ����Ӧͨ��X������Ϊ����������A��B��C���������з�Ӧ��ʼǰ���Լ��ֱ��ǣ�A�٣�B�ޣ�C�ڣ�����ţ���
��ϡ���� ��Ũ���� ��ϡ���� ��ʯ��ʯ �ݴ��� ��п��
��2��ʵ�鿪ʼʱ��������ͨ��X���壬�ټ��ȷ�Ӧ��������Ƿ�ֹ����������H2�������ϱ�ը��
����Ӧ���������߾ƾ��ƣ���Ӧ�ܼ������У���ԭ����Mg��SiO2�ķ�Ӧ�Ƿ��ȷ�Ӧ��
��3����Ӧ��������ȴ������ʱ������Ӧ��Ļ�����м���ϡ���ᣬ�ɹ۲쵽�����Ļ��ǣ������������ԭ���û�ѧ����ʽ��ʾΪ����Mg2Si+4HCl=2MgCl2+SiH4������SiH4+2O2�TSiO2+2H2O��
��4�������ƵõĹ��������ʣ���SiO2�ȣ����ƴֹ裬����������������̽����ᴿ

������ʵ��������£�
| ���� | Si | SiCl4 |
| �е�/�� | 2355 | 57.6 |
 �������ȶ�����ˮ������᪻���Ӧ��һ������Ԫ�ؼ�̬���ߣ�һ���ֽ��ͣ�����Ӧ�漰�ļ������ʵ��۷е������
�������ȶ�����ˮ������᪻���Ӧ��һ������Ԫ�ؼ�̬���ߣ�һ���ֽ��ͣ�����Ӧ�漰�ļ������ʵ��۷е������| ���� | S | CS2 | CCl4 | S2Cl2 |
| �е�/�� | 445 | 47 | 77 | 137 |
| �۵�/�� | 113 | -109 | -23 | -77 |
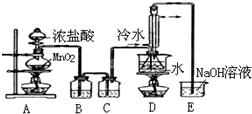
��1��д��Aװ���з�����Ӧ�����ӷ���ʽMnO2+4H++2Cl-$\frac{\underline{\;\;��\;\;}}{\;}$Mn2++Cl2��+2H2O��
��2��ʵ��������ͨ������36.5%��Ũ��Һ������ϡ�����������ϡ���ỹԭ��������Ӧ���ѣ�
��3��D����������������������˫�����ã�������ȴװ�ÿ�Ӧ�������и��л�ѧ��ACDʵ�飮
A��ʯ�ͷ��� B���Ʊ���ϩ
C����ȡ�������� D����ȡ�屽
��4��Bװ����ʢ�ŵ��DZ���ʳ��ˮ����Ӧ���������ƿ�ڻ�����з������Ʒ�ķ���������D�в�����ˮԡ���ȵ�ԭ����ʹCS2ƽ���������������S2Cl2������
��5��Aװ������װ��ʱ������װ��װ����Ϻ�Ӧ�Ƚ��������Լ�飬�������Լ���ʵ����ϣ����װ��ʱ��Ӧ�Ƚ�E�г������ƿ�Һ�棬Ŀ���Ƿ�ֹ������
��6��ʵ������У���ȱ��Cװ�ã����ֲ�Ʒ��ʴ���壬���ָ������ԭ����û�ѧ����ʽ��ʾΪ2S2Cl2+2H2O=3S��+SO2��+4HCl����ʵ����ϣ�����ʣ��Ũ���ᵹ��E�ձ�����������β��������������Һ���ʱ����������������ɫ�̼�����������������������ԭ����ClO-+2H++Cl-=Cl2��+H2O���������ӷ���ʽ��ʾ��
| ������� | �¶ȣ��棩 | ��ʼ���ʵ�����mol�� | ƽ�����ʵ�����mol�� | |
| CH3OH��g�� | CH3OCH3��g�� | H2O��g�� | ||
| �� | 390 | 0.20 | 0.080 | 0.080 |
| �� | 390 | |||
| �� | 230 | 0.20 | 0.090 | 0.090 |
��1����Ӧ�ġ�H��O ����������������жϵ�������Ͷ������ͬʱ���¶ȸߵ�������ƽ��ʱ�����ѵ����ʵ���С��˵����ӦΪ���ȷ�Ӧ����HС��0��
��2������I����ƽ�������ʱ��Ϊ20s����Ӧ����v��CH3OH��Ϊ0.004mol/��L•s����390��ʱ�÷�Ӧ��ƽ�ⳣ��K1=4������ֵ����
��3������II��ƽ��ʱ��ѹǿ������I��������CH3OH���������������I�е���ͬ��CH3OH��ʼ�����ʵ���Ϊ0.40mol��ƽ��ʱCH3OH��g�����������Ϊ20%��
��4��390�棬����3L�����г���0.9mol CH3OH��g����0.6mol CH3OCH3��g����0.3molH2O��g��������ʼʱ�÷�Ӧ����V����V�森���������������=����
 ��
��