��Ŀ����
18��PbI2������ɫ��ĩ����������������̫���ܵ�ص�������--�װ�Ǧ���ԭ�ϣ��ϳ�PbI2��ʵ��������ͼ1��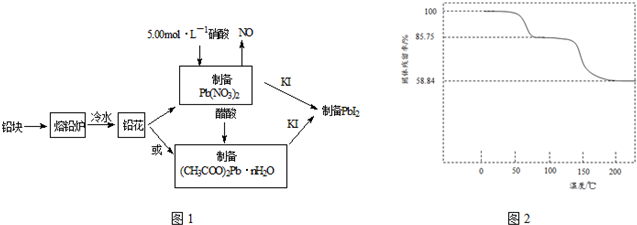
��1����Ǧ���Ƴ�Ǧ����Ŀ������������ĽӴ�������ӿ��ܽⷴӦ���ʣ�
��2��31.05gǦ����5.00mol•L-1�������ܽ⣬����������5.00mol•L-1����80mL��
��3��ȡһ��������CH3COO��2Pb•nH2O��Ʒ��N2�����м��ȣ������Ʒ��������ʣ���$\frac{������Ʒ��ʣ������}{������Ʒ����ʼ����}$��100%�����¶ȵı仯��ͼ2��ʾ����֪����Ʒ��75��ʱ����ȫʧȥ�ᾧˮ����
�٣�CH3COO��2Pb•nH2O�нᾧˮ��Ŀn=3������������
��100��200���ֽ����ΪǦ���������һ���л������л���ΪC4H6O3��д����ʽ����
��4����ȡһ��������PbI2���壬������ˮ���Ƴ�����ʱ�ı�����Һ��ȷ��ȡ25.00mL PbI2������Һ�ִμ��������ӽ�����֬RH�У�������2RH��s��+Pb2+��aq��=R2Pb��s��+2H+��aq��������ƿ��������Һ�����������ˮ��ϴ��֬������Һ�����ԣ���ϴ��Һ�ϲ�����ƿ�У�����2��3�η�̪��Һ����0.002500mol•L-1NaOH��Һ�ζ������ζ��յ�ʱ��ȥ�������Ʊ���Һ20.00mL��������ʱPbI2 ��KspΪ4.000��10-9��
��5��̽��Ũ�ȶԻǻ�Ǧ�����ܽ�ƽ���Ӱ�죮
�û�ѧС��������ṩ�Լ��������ʵ�飬��˵��Ũ�ȶԳ����ܽ�ƽ���Ӱ�죮
�ṩ�Լ���NaI������Һ��NaCl������Һ��FeCl3 ������Һ��PbI2������Һ��PbI2����Һ��
��Ϣ��ʾ��Pb2+��Cl-���γɽ��ȶ���PbCl42-�����ӣ�
����д�±��Ŀհ״���
| ʵ������ | ʵ�鷽�� | ʵ������ԭ����� |
| �ٻ�����Ũ�������ƽ���Ӱ�� | ȡPbI2������Һ������һ֧�Թ��У��ٵ��뼸��NaI������Һ | ������Һ��c��I-������ʹQ������PbI2��Ksp |
| ��Ǧ����Ũ�ȼ�С��ƽ���Ӱ�� | ȡPbI2����Һ������һ֧�Թ��У��ټ�������NaCl������Һ | ����ɫ������ʧ ԭ���γ�PbCl42-��������Һ��c��Pb2+����С��ʹQcС��PbI2��Ksp |
| ��Ǧ���Ӻ͵�����Ũ�ȶ���С��ƽ���Ӱ�� | ��PbI2����Һ�е��뼸��FeCl3 ������Һ | ����ɫ������ʧ д����Ӧ�����ӷ���ʽ�� PbI2+2Fe3++4Cl-=PbCl42-+2Fe2++I2 |
���� �÷�Ǧ��Ϊԭ�Ϻϳ�PbI2�����̣�Ϊ���������ᷴӦ�ĽӴ�������ӿ��ܽⷴӦ���ʣ���Ǧ���Ƴ�Ǧ����;��һ��3Pb+8HNO3=3Pb��NO3��2+2NO��+4H2O��Pb��NO3��2+KI=PbI2��+KNO3��;������Pb��NO3��2+2CH3COOH+nH2O=��CH3COO��2Pb•nH2O+2HNO3����CH3COO��2Pb•nH2O+2KI=PbI2��+2CH3COOK+nH2O��
��1����Ӧ��Ӵ����Խ���䷴Ӧ����Խ�죻
��2��Ǧ�ܽ���ϡ����ķ�Ӧ����ʽΪ3Pb+8HNO3=3Pb��NO3��2+2NO��+4H2O������Pb������֮��Ĺ�ϵʽ�������ĵ����
��3���ٿɼ�����ʼ��������Ϊ100g������ȵ�75��ʱ��ȫʧȥ�ᾧˮ���������ǹ������������������ˮ�����ʵ���������Ǧ�����ʵ������������ߵ����ʵ���֮�ȿ�ȷ��n��ֵ��
�ڴ���Ǧ�������ȷֽ�ʣ�����ΪǦ��������ɸ���ʣ�����������Ǧ��̼ԭ���غ���ȷ���л����Ħ���������Ʋ��л���ķ�����ɣ�
��4����������к͵�ԭ���ɼ������Һ�е�c��H+��������Ϲ�ϵʽȷ����Һ��c��Pb2+�����ټ���Ksp��
��5��Ӱ�컯ѧƽ���ƶ�������-Ũ�ȣ�����Ӧ���С�����Ũ�ȣ�����������У������������С��Ӧ�Ũ��ƽ��������У�
��� �⣺��1����Ӧ��Ӵ����Խ���䷴Ӧ����Խ�죬��Ǧ���Ƴ�Ǧ������Ӧ��Ӵ��������Ӧ���ʾ��죬
�ʴ�Ϊ����������ĽӴ�������ӿ��ܽⷴӦ���ʣ�
��2��31.05gǦ�����ʵ���Ϊ$\frac{31.05g}{207g/mol}$=0.15mol�����ݷ�Ӧ����ʽ3Pb+8HNO3=3Pb��NO3��2+2NO��+4H2O��֪���ĵ�n��HNO3��=0.15mol��$\frac{8}{3}$=0.4mol����������Һ�����Ϊ0.4mol��5.00mol•L-1=0.08L=80.0mL��
�ʴ�Ϊ��80��
��3���ټ�����Ʒ����ʼ����Ϊ100�ˣ����ݹ�������ʵĹ�ʽ��֪��75��ʱʣ�����Ϊ87.75�ˣ����ɵ�ˮ����Ϊ100g-87.75g=12.25g�������Ǧ��ˮ�����ʵ���֮��Ϊ$\frac{85.75g}{325g/mol}$��$\frac{12.25g}{18g/mol}$=1��3����n=3��
�ʴ�Ϊ��3��
��Ǧ������������Ϊ58.84�ˣ�����Ǧ�����ʵ���Ϊ=$\frac{85.75g}{325g/mol}$=$\frac{85.75}{325}$mol������Ǧԭ���غ㣬Ǧ�������PbOx�������ʵ���Ϊ$\frac{85.75}{325}$mol�����������Ħ������Ϊ$\frac{58.84g}{\frac{85.75}{325}mol}$=223g/mol��ΪPbO���л��������Ϊ85.75g-58.84g=26.91g�����л��������Ӧ�����ĸ�̼ԭ�ӣ����ʵ���Ϊ$\frac{85.75}{325}$mol��Ħ������=$\frac{26.91g}{\frac{85.75}{325}mol}$=102g/mol������ԭ���غ��֪�л���ķ���ʽΪC4H6O3��
�ʴ�Ϊ��C4H6O3��
��4��n��H+��=n��NaOH��=0.002500 mol•L-1��20.00mL��10-3L•mL-1=5.000��10-5mol
n[Pb2+��aq��]=$\frac{1}{2}$n��H+��=2.500��10-5mol
c��Pb2+��=$\frac{2.500��1{0}^{-5}mol}{25.00mL��1{0}^{-3}L/mL}$=1.000��10-3 mol•L-1
Ksp��PbI2��=c��Pb2+��•c2��I-��=4c3��Pb2+��=4����1.000��10-3��3=4.000��10-9��
��4.000��10-9��
��5����ȡPbI2����Һ������һ֧�Թ��У��ټ�������NaCl������Һ���γ�PbCl42-��������Һ��c��Pb2+����С��ƽ�������ƶ����⻯Ǧ�ܽ⣬���»�ɫ������ʧ��
����PbI2����Һ�е��뼸��FeCl3������Һ������������ԭ��ӦPbI2+2Fe3++4Cl-=PbCl42-+2Fe2++I2���⻯Ǧ�ܽ⣬���»�ɫ������ʧ���ʴ�Ϊ��
| ʵ������ | ʵ�鷽�� | ʵ������ԭ����� |
| ȡPbI2����Һ������һ֧�Թ��У��ټ�������NaCl������Һ | ����ɫ������ʧ ԭ���γ�PbCl42-��������Һ��c��Pb2+����С��ʹQcС��PbI2��Ksp | |
| Ǧ���Ӻ͵�����Ũ�ȶ���С��ƽ���Ӱ�� | PbI2+2Fe3++4Cl-=PbCl42-+2Fe2++I2 |
���� ���⿼���Ʊ�ʵ�鷽����ƣ�Ϊ��Ƶ���㣬�漰��Ӧ����Ӱ�����ء��������ܽ�ƽ�⡢������ԭ��Ӧ���ܶȻ������֪ʶ�㣬���ؿ���ѧ����������������ע�⣨2����5������йؼ���ͷ�����Ϊ�ѵ㣬ע������Ӻ��������ܷ���������ԭ��Ӧ��

 ���ݼ���ϵ�д�
���ݼ���ϵ�д�| A�� | Ksp��CaF2�����¶Ⱥ�Ũ�ȵı仯���仯 | |
| B�� | ��1 L0.2 mol•L-1 HF��Һ�м���1 L 0.2 mol•L-1 CaCl2��Һ��û�г������� | |
| C�� | AgCl������ˮ������ת��ΪAgI | |
| D�� | ����AgCl����NaI��Һ�п�ʼת��ΪAgI��NaIŨ�ȱ��벻����$\frac{1}{\sqrt{1.8}}$��10-11 mol•L-1 |
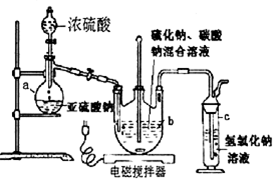
�ٿ�����Һ©����ʹ�����������£��ʵ����������У�ʹ��Ӧ������SO2����Ͼ��ȵ�ͨ��Na2S��Na2CO3�Ļ����Һ�У�ͬʱ������Ž�����������
����������������ʧ��������Һ��pH�ӽ�7ʱ��ֹͣͨ��SO2���壮
�۳������õ���Һ��ת�����������У�ˮԡ����Ũ����ֱ����Һ������־�Ĥ��
����ȴ�ᾧ�����ˡ�ϴ�ӣ�
�ݽ������������У���40��45�����Ҹ���50��60min��������
��ش��������⣺
��l������a��������������ƿ��
��2���������������pHֵС��7������ʻ��½����������ӷ���ʽ����ԭ��S2O32-+2H+=S��+H2O+SO2����
��3��������в��ܽ���Һ�������ɵ�ԭ�������ɻ�ʹ�����������ˮ���ֽ⣻��Ĥͨ������Һ������ֵ�ԭ������Ϊ��Һ�����¶Ƚϵͣ�
��4���������ϴ����������ƾ��������Լ��Ľṹʽ��
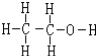 ��
����5��Ϊ�����ƵõIJ�Ʒ�Ĵ��ȣ���ʵ��С���ȡ5��0�˵IJ�Ʒ���Ƴ�250mL�����������Һ�����ü�ӵ������궨����Һ��Ũ�ȣ�����ƿ�м���25mL 0.0lmol•L-1KIO3��Һ�������������KI���ữ���������з�Ӧ��5I-+IO3-+6H+=3I2+3H2O���ټ��뼸�ε�����Һ������������Na2S2O3��Һ�ζ���������Ӧ��I2+2S2O32-=2I-+S4O62-������ɫ��ȥH����Ӳ���ɫʱ����ζ��յ㣮ʵ���������±���
| ʵ����� | 1 | 2 | 3 |
| Na2S2O3��Һ�����mL�� | 19.98 | 20.02 | 21.18 |
A���ζ���ĩ��Na2S2O3��Һ��ϴ B���ζ��յ�ʱ���Ӷ���
C����ƿ������ˮ��ϴ D���ζ��ܼ��촦�ζ�ǰ�����ݣ��ζ��յ㷢�����ݣ�

��1������������漰��Ӧ�����ӷ���ʽ��6H++Al2O3�T2Al3++3H2O��6H++Fe2O3�T2Fe3++3H2O��
��2����֪��
�����������������pH
| Al��OH��3 | Fe��OH��3 | |
| ��ʼ����ʱ | 3.4 | 1.5 |
| ��ȫ����ʱ | 4.7 | 2.8 |
���ݱ������ݽ��ͼ���X����ҪĿ�ģ�������ҺpH��Fe3+��ȫ��������������Ҫ�������ʵĻ�ѧʽ��Fe��OH��3��SiO2��
��3����֪������Һ��PAC�ķ�ӦΪ2Al3++m��6-n�� Cl-+mn H2O?[Al2��OH��nCl6-n]m+mn H+��
��̼��Ƶ�����Һ��pHʱ��Ҫ�Ͽ�pH�Ĵ�С��pHƫС��ƫ��Һ��PAC�IJ��ʶ��ή�ͣ������pHƫСҺ��PAC���ʽ��͵�ԭ��pHƫСʱ������ƽ��2Al3++m��6-n�� Cl-+mn H2O?[Al2��OH��nCl6-n]m+mn H+ �����ƶ�����PAC��
��4��Ũ���ۺϵõ���PAC��Һ�������ĸ�����̬��Ҫ������
Ala--Al3+������̬��
Alb--[Al2��OH��nCl6-n]m�ۺ���̬��
Alc--Al��OH��3������̬
ͼ1ΪAl����̬�ٷ������¶ȱ仯�����ߣ�ͼ2Ϊ��PAC��Һ����������Ũ��AlT���¶ȱ仯�����ߣ�

��50-90��֮���Ʊ���Һ��PAC�У��ۺ���̬��������࣮
�ڵ�T��80��ʱ��AlT���Խ��͵�ԭ�����¶����ߣ�����Һ��PAC��Al��OH��3����ת����
 ijʵ��С������ͼ��ʾ��װ���Ʊ�һ�����ױ������������ױ��Ͷ������ױ�����
ijʵ��С������ͼ��ʾ��װ���Ʊ�һ�����ױ������������ױ��Ͷ������ױ�������Ӧԭ����

ʵ�鲽�裺������Ũ������Ũ���ᣨ�������1��3���Ļ����Һ�������ᣩ40mL��
��������ƿ�м���15mL��13g���ױ���
�۰�ͼ��ʾװ��ҩƷ����װ������������
��������ƿ�м�����ᣬ�����Ͻ��裨��������������ȥ����
�ݿ����¶�ԼΪ50�棬��Ӧ��Լ10min������ƿ���д�������ɫ��״Һ����֣�
�����һ�����ױ���
ʵ���п����õ������ݣ�
| �ܶ�g•cm-3 | �е�/�� | �ܽ��� | |
| �ױ� | 0.866 | 110.6 | ������ˮ�������������ױ� |
| ��Ʒ1 | 1.286 | 237.7 | ������ˮ��������Һ���� |
| ��Ʒ2 | 1.162 | 222 | ������ˮ��������Һ���� |
��1������40mL����IJ����ǽ�30mLŨ����ע���ձ��У������ձ��ڱ�ע��10mLŨ���ᣬ�ӱ߽��裻
��2����ʵ����Ũ����������Ǵ�������ˮ����
��3��װ��������Aʹ��ǰ����ϴ�ɾ�����©��
��4�������Ʒ�ķ������£�

����2����IJ�����������6�֣�
��5�������յõ���Ʒ1�Ͳ�Ʒ2��������Ϊ17.42g����һ�����ױ����ܲ�����89.99%��������λС����
��1������ͼIװ����ȡ��������ƿ�п�ѡ����Լ����������ƹ��壨��Ũ��ˮ���ʯ�һ�Ũ��ˮ����ʯ�ң��ȣ�
��2���Ʊ���������淋�װ������ͼ����ʾ����NH3��CO2ͨ�����Ȼ�̼�У����Ͻ����ϣ����ɵİ�������淋�С����������CCl4�У���������϶�ʱ��ֹͣ�Ʊ���

ע��CCl4��Һ��ʯ����Ϊ���Խ��ʣ�
��%2ͼI�еμ�Һ������������Ƿ�Һ©����Һ��ʯ������ƿ��������ͨ���۲����ݣ�����NH3��CO2ͨ���������ͨ���۲����ݣ�����NH3��CO2�ķ�Ӧ���ʣ����������ñ�ˮ��ȴ��ԭ���ǽ����¶ȣ���߷�Ӧ��ת���ʣ����¶ȣ���ֹ��Ӧ������ɲ���ֽ⣩��
�ڴӷ�Ӧ��Ļ�����з������Ʒ��ʵ�鷽���ǹ��ˣ���д�������ƣ���Ϊ�˵õ������Ʒ��Ӧ��ȡ�ķ�����b����дѡ����ţ���
a����ѹ���Ⱥ�� b����ѹ40�����º�� c����ѹ���Ⱥ��
��3���Ƶõİ�������刺��ܺ���̼����李�̼����е�һ�ֻ����֣�
����Ʒ��������гɷ�̽��������д���пո�
��ѡ�Լ�������ˮ��ϡHNO3��BaCl2��Һ��Ba��OH��2��Һ��AgNO3��Һ��ϡ���ᣮ
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1��ȡ����������Ʒ���Թ��У���������ˮ�������ܽ⣮ | �õ���ɫ��Һ |
| ����2�����Թ��м��������BaCl2��Һ�����ã� | ��Һ����ǣ���֤�������к��У�NH4��2CO3�� |
| ����3��ȡ����2���ϲ���Һ���Թ��м���������Ba��OH��2��Һ�� | ��Һ������ǣ���֤�������в�����NH4HCO3�� |
| H2C2O4 | ��ɫ���� | K1=5.9��10-2��K2=6.4��10-5��������ˮ���Ҵ� |
| Na2C2O4 | ��ɫ���� | ����ˮ��pH=7.2���������Ҵ� |
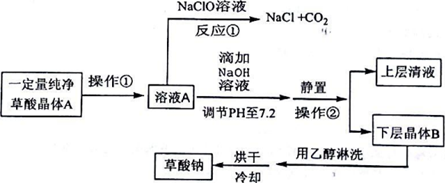
��ش��������⣺
��1��д����Ӧ�ٵĻ�ѧ����ʽH2C2O4+NaC1O=NaC1+2CO2��+H2O������������л�ԭ�ԣ�
��2������ҺA�м���NaOH��Һ����ʼ�μ��ٶ�Ҫ������Щ����Ŀ���������кͷ�Ӧ���ȣ����´ٽ���Ӧ��������Ӧ���ʣ����÷�Ӧ�ﵽ�յ�ʱ�Ļ�ѧ����ʽΪH2C2O4+2NaOH=Na2C2O4��+2H2O��
��3�������ڵ������ǹ��ˣ����Ҵ���ϴ����B��Ŀ���dz�ȥ�������ˮ�ּ�����ʧ
��4����0.01000mol/L�ĸ��������Һ�ζ�25.00mLijŨ�ȵIJ�������Һʱ����Ҫ����������ϡ���ᣬ��������ӦΪ��5C2O42-+2MnO4-+16H+�T2Mn2++10CO2��+8H2O�����������̫�࣬��������������ɲ�������Ͳ��ᣬʹ��Һ�в��������Ũ�Ƚ��ͣ�������Ӧ���ʣ�������������ʽ�ݶ��ܣ����ʽ����ʽ���������ﵽ��Ӧ�յ�ʱ����������Һ����dz��ɫ��30s�ڲ���ɫ����ô�ʱ�����������������Һ20.00mL����ò�������ҺŨ��Ϊ0.0200mol/L��

| A�� | ��װ�ü���ȡ���� | |
| B�� | ��װ��������FeBr3��Һ�е������� | |
| C�� | ��װ�ñ���Һʱ�ȴ��¿ڷų�ˮ�࣬�ٴ��Ͽڵ����л��� | |
| D�� | ��װ�ö�����Һ���ˮ���������ɣ��������Ƶ���ˮFeCl3 |