��Ŀ����
20��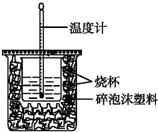 ��1����֪H+��aq��+OH-��aq���TH2O��l����H=-57.3kJ•mol-1����50mL0.50mol•L-1������50mL0.55mol•L-1NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺
��1����֪H+��aq��+OH-��aq���TH2O��l����H=-57.3kJ•mol-1����50mL0.50mol•L-1������50mL0.55mol•L-1NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺�ٴ�ʵ��װ���Ͽ���ͼ����ȱ�ٵ�һ�ֲ��������ǻ��β����������
��Ϊ�˲ⶨ��Ӧ���к��ȣ�����ʱ������Ҫ��������A
a�����Ũ�Ⱥ���� b�����Ũ�Ⱥ������ c��������
d����Ӧ����Һ�������� e������ˮ�����ʵ���
f����Ӧǰ���¶ȵı仯�� g����������ʱ��
A��abcf B��acde
C��cdef D��ȫ��
����ͨ����ʵ��ⶨ�к��ȵġ�H��������������-57.3kJ•mol-1����ԭ����ܣ�ʵ�����������������ɢʧ��
������ͬŨ�Ⱥ�����İ�ˮ��NH3•H2O������NaOH��Һ��������ʵ�飬��õ��к��ȵ���ֵ��ƫС���ƫ����ƫС������Ӱ�족����
��2����֪�Ȼ�ѧ����ʽ��
2H2O��l���T2H2��g��+O2��g����H=+571.6kJ•mol-1
2H2��g��+O2��g���T2H2O��g����H=-483.6kJ•mol-1
��1gҺ̬ˮ��Ϊ��̬ˮʱ�����������仯������������
�ٷų������������������� ��2.44kJ�� ��4.88kJ�� ��88kJ��������ȷ���Ǣں͢�
��3����֪CH3OH��l����HCHO��g����ȼ���ȡ�H�ֱ�Ϊ-726.64kJ•mol-1 ��-563.58kJ•mol-1����CH3OH��l����O2��g����Ӧ����HCHO��g����H2O��l�� ���Ȼ�ѧ��Ӧ����ʽ��CH3OH��l��+$\frac{1}{2}$O2��g��=HCHO��g��+H2O��l����H��-163.06kJ•mol-1��
���� ��1���ٸ������ȼƵĹ������жϸ�װ�õ�ȱ��������
�ڸ����к��ȵļ��㹫ʽ��H=-$\frac{cm��T}{n}$��ȷ��ʵ������Ҫ�����ݣ�
��������Ч�����ã�������ɢʧ����õ��к�����ֵ�����С��
�ܸ���������ʵ������ȷ�����
��2����1g Һ̬ˮ�����̬ˮʱ����Ҫ���ȣ����ݸ�˹���ɼ���õ�Һ̬ˮ�仯Ϊ����ˮ���յ������������Ȼ�ѧ����ʽ����1gˮ�仯Ϊ��̬ˮ���յ�������
��3������ȼ���ȵĸ�����дCH3OH��l����HCHO��g����ȼ���ȷ���ʽ�����ݸ�˹���������㻯ѧ��Ӧ���ʱ䣮
��� �⣺�������ȼƵĹ����֪��װ�õ�ȱ�������ǻ��β����������
�ʴ�Ϊ�����β����������
���ɷ�Ӧ�ȵļ��㹫ʽ��H=-$\frac{cm��T}{n}$��֪����Ӧ���к��ȼ���ʱ��������Ҫ�������У������ݣ����Ũ�Ⱥ���������Ũ�Ⱥ��������Ӧǰ���¶ȱ仯��T��Ȼ��������Ӧ����Һ������������ˮ�����ʵ�����
�ʴ�Ϊ��A��
��������Ч�����ã�������ɢʧ����õ��к�����ֵ�����С����H����-57.3kJ•mol-1��
�ʴ�Ϊ��ʵ�����������������ɢʧ��
�ܰ�ˮΪ����������Ϊ���ȹ��̣�����ͬŨ�Ⱥ�����İ�ˮ��NH3•H2O������NaOH��Һ��������ʵ�飬��õ��к��ȵ���ֵƫС���ʴ�Ϊ��ƫС
��2����1g Һ̬ˮ�����̬ˮʱ����Ҫ���ȣ����ݸ�˹���ɼ���õ�Һ̬ˮ�仯Ϊ�������յ�������
��֪�Ȼ�ѧ����ʽ��
��2H2O��l��=2H2��g��+O2��g����H1=+571.6kJ/mol��
��2H2��g��+O2��g��=2H2O��g����H2=-483.6kJ/mol��
���ݸ�˹���ɢ�+�ڵõ�2H2O��l��=2H2O��g����H3=+88kJ/mol
�����Ȼ�ѧ����ʽ���㣬��1g Һ̬ˮ�����̬ˮʱ����Ҫ����2.44kJ��
���Ԣڢ���ȷ��
�ʴ�Ϊ���ںۣ͢�
��3����֪CH3OH��l����ȼ����Ϊ-726.64kJ•mol-1����CH3OH��l��+$\frac{3}{2}$O2��g��=CO2��g��+2H2O��l����H=-726.5kJ•mol-1��
��֪HCHO��g����ȼ����Ϊ-726.64kJ•mol-1����HCHO��g��+O2��g��=CO2��g��+H2O��l����H=-563.58kJ•mol-1��
���ݸ�˹���ɣ���-�ڵõ���CH3OH��l��+$\frac{1}{2}$O2��g��=HCHO��g��+H2O��l������H=-726.5kJ•mol-1-��-563.58kJ•mol-1��=-163.06 kJ•mol-1��
�ʴ�Ϊ��CH3OH��l��+$\frac{1}{2}$O2��g��=HCHO��g��+H2O��l����H��-163.06 kJ•mol-1��
���� ���⿼�黯ѧ�������ܣ������ڲⶨ��Ӧ�ȵ�ԭ������������ȼ���ȵĶ����˹���ɵ�Ӧ�ã��Ѷ��еȣ�ע�������к��ȡ�ȼ���ȵĸ��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | ���ƺϽ�Ͷ�뵽�����Ȼ�ͭ��Һ�У��϶���������ͭ����Ҳ������ͭ���� | |
| B�� | ���ƺϽ���Ͷ��һ����ˮ�пɵ���ɫ��Һ����n��Al����n��Na�� | |
| C�� | ���ƺϽ�Ͷ������ˮ�У����ų���H2Խ�࣬��������������Խ�� | |
| D�� | ���ƺϽ�Ͷ�����������У����ų���H2Խ�࣬��������������Խ�� |
| A�� | ���ʹ��ǽ�̫����ת��Ϊ���ܵij��ò��� | |
| B�� | ���ø����������ҩ�ý��һ�����彡�����Σ�� | |
| C�� | ������������θ����кͼ� | |
| D�� | ����ˮ��ʱ�������������ԵĽ������ӣ�����Ư�� |
| A�� | 0.05 mol/L | B�� | 0.01 mol/L | C�� | 0.02 mol/L | D�� | 0.03 mol/L |
| A�� | 0.1mol/L NH4Cl��Һ��0.05mol/L NaOH��Һ�������Ϻ����õļ�����Һ�У�c��Cl-����c��Na+����c��NH${\;}_{4}^{+}$����c��OH-����c��H+�� | |
| B�� | pH=2��HA��Һ��pH=12��MOH��Һ�������ϣ�c��M+��=c��A-����c��OH-��=c��H+�� | |
| C�� | �����ʵ�����NaClO��NaHCO5�����Һ�У�c��HClO��+c��ClO-���Tc��HCO3-��+c��H2CO3��+c��CO32-�� | |
| D�� | ij��Ԫ�������ʽ��NaHA��Һ��c��Na+��+c��H+��=c��OH-��+c��HA-��+c��A2-�� |
| �� Ⱦ ָ �� | ||
| SO2 | NO2 | PM10 |
| 49 | 21 | 23 |
��1��������Ҫ��Ⱦ����SO2����������״��Ϊ�����Ⱦ����д���š�����������������Ⱦ�������ж���Ⱦ������
��2������PM10��ָ���ǿ���������� ����д��Ⱦ���Ӧ�����ƣ�
��3�������������Ϊ��Դ��Ҫ�ǻ�ʯȼ�ϵ�ȼ�գ��ڳ����д����еĵ���������Ҫ��������β����
��4��SO2��NO2��PM10�����������ܹ��γ��������SO2��NO2��
 2010���ڣ����꣩�ĵ�����ũ��ʦ�������ʻ�ʩ����S-�տ��ؼ������ӽṹ��ͼ������ʹ�ʻ���ʱʢ��������˵������ȷ���ǣ�������
2010���ڣ����꣩�ĵ�����ũ��ʦ�������ʻ�ʩ����S-�տ��ؼ������ӽṹ��ͼ������ʹ�ʻ���ʱʢ��������˵������ȷ���ǣ�������| A�� | S-�տ��صķ���ʽΪC15 H20 O4 | |
| B�� | S-�տ��ؼ��ܷ����Ӿ۷�Ӧ�����ܷ������۷�Ӧ | |
| C�� | 1 mol S-�տ�������ܺͺ�1 mol NaOH��ˮ��Һ������Ӧ | |
| D�� | S-�տ��ؼȿ�����FeCl3��Һ������ɫ��Ӧ���ֿ���ʹ����KMnO4��Һ��ɫ |
 ��18.4mol•L-1��ŨH2SO4����100mLŨ��Ϊ1mol•L-1��ϡH2SO4��������ɷ�Ϊ���¸�����
��18.4mol•L-1��ŨH2SO4����100mLŨ��Ϊ1mol•L-1��ϡH2SO4��������ɷ�Ϊ���¸�����