��Ŀ����
19������A��B��C��D��E��������Һ���ֱ���K+��NH4+��Ag+��Ba2+��Al3+��Cl-��Br-��CO32-��SO42-��NO3-�е��������������Ӹ�һ����ɣ��������������������Ӹ�����ͬ������֪����A+B���ס�����A+C���ס�����A+D���ס�����B+C���ס�+���������A����Һ��lgc��H+��=lgc��OH-������B����Һ��c��H+��=1��10-12mol/L����C��D��E������Һ��pH��7����1������������ʵд����ѧʽ��
ABaCl2��BK2CO3��CAl2��SO4��3��DAgNO3��ENH4Br��
��2��д���٢ڢܸۢ�����Ӧ�����ӷ���ʽ��
Ba2++CO32-�TBaCO3����Ba2++SO42-�TBaSO4����Ag++Cl-�TAgCl����2Al3++3CO32-+3H2O�T2Al��OH��3��+3CO2����
���� Ag+����Cl-��Br-��SO42-��CO32-�������棬��ΪAgCl��Ag2SO4��Ag2CO3��Ϊ��ɫ������AgBrΪdz��ɫ���������������к���AgNO3��
�ɢ���B��C��Ӧ���ɰ�ɫ���������壬������������ֻ��ΪAl3+��CO32-������˫ˮ�ⷴӦ��2Al3++3CO32-+3H2O�T2Al��OH��3��+CO2����
�ɢ�֪B����Һ�ʼ��ԣ�����Ŀ������������ֻ��ΪCO32-Ϊ��������ӣ�������Ϊǿ���������ӣ�ǿ��������������Ŀ��������������ʵ�ˮ��Һ�����Ի����ԣ���B�к���CO32-����֪C�к���Al3+�������ӹ����֪��B�к��е�������ΪK+��NH4+��
�������������������Ӹ�����ͬ���ɢ�A+B���ס�����֪A����������B��CO32-���ɰ�ɫ��������ΪK+��NH4+�������Ӿ��������ɳ�������A��������ֻ��ΪAg+��Ba2+�е�һ�֣��ɢݿ�֪A����Һ�����ԣ���A�к���Ba2+����ΪAgNO3��Һ�����ԣ���A��������ΪCl-��Br-����Ϣ�A+C���ס�����֪A�������ӣ�Ba2+����C�����������ɰ�ɫ��������Ϊ��Ŀ��CO32-��������������Ӿ�����Al3+��Ӧ����C��������ΪSO42-������֪CΪAl2��SO4��3���ɢ�A+D���ס�����֪A����������D�����������ɰ�ɫ��������ΪBa2+����Ŀ��CO32-��SO42-��������������Ӳ���Ӧ����DΪAgNO3��AΪBaCl2���ɢ���C��D��E������Һ��pH��7����֪EΪNH4Br����ΪKBr��Һ�����ԣ���BΪK2CO3��
��� �⣺��1��Ag+����Cl-��Br-��SO42-��CO32-�������棬��ΪAgCl��Ag2SO4��Ag2CO3��Ϊ��ɫ������AgBrΪdz��ɫ���������������к���AgNO3��
�ɢ���B��C��Ӧ���ɰ�ɫ���������壬������������ֻ��ΪAl3+��CO32-������˫ˮ�ⷴӦ��2Al3++3CO32-+3H2O�T2Al��OH��3��+CO2����
�ɢ�֪B����Һ�ʼ��ԣ�����Ŀ������������ֻ��ΪCO32-Ϊ��������ӣ�������Ϊǿ���������ӣ�ǿ��������������Ŀ��������������ʵ�ˮ��Һ�����Ի����ԣ���B�к���CO32-����֪C�к���Al3+�������ӹ����֪��B�к��е�������ΪK+��NH4+��
�������������������Ӹ�����ͬ���ɢ�A+B���ס�����֪A����������B��CO32-���ɰ�ɫ��������ΪK+��NH4+�������Ӿ��������ɳ�������A��������ֻ��ΪAg+��Ba2+�е�һ�֣��ɢݿ�֪A����Һ�����ԣ���A�к���Ba2+����ΪAgNO3��Һ�����ԣ���A��������ΪCl-��Br-����Ϣ�A+C���ס�����֪A�������ӣ�Ba2+����C�����������ɰ�ɫ��������Ϊ��Ŀ��CO32-��������������Ӿ�����Al3+��Ӧ����C��������ΪSO42-������֪CΪAl2��SO4��3���ɢ�A+D���ס�����֪A����������D�����������ɰ�ɫ��������ΪBa2+����Ŀ��CO32-��SO42-��������������Ӳ���Ӧ����DΪAgNO3��AΪBaCl2���ɢ���C��D��E������Һ��pH��7����֪EΪNH4Br����ΪKBr��Һ�����ԣ���BΪK2CO3��
�ʴ�Ϊ��BaCl2��K2CO3��Al2��SO4��3��AgNO3��NH4Br��
��2���ٵ����ӷ���ʽΪ��Ba2++CO32-�TBaCO3����
�ڵ����ӷ���ʽΪ��Ba2++SO42-�TBaSO4����
�۵����ӷ���ʽΪ��Ag++Cl-�TAgCl����
�ܵ����ӷ���ʽΪ��2Al3++3CO32-+3H2O�T2Al��OH��3��+3CO2����
�ʴ�Ϊ����Ba2++CO32-�TBaCO3������Ba2++SO42-�TBaSO4������Ag++Cl-�TAgCl������2Al3++3CO32-+3H2O�T2Al��OH��3��+3CO2����
���� ���������ƶϣ��ؼ���ȷ����֮�䷢���ķ�Ӧ�Լ�Ԫ�ػ��������ʵȣ����ؿ���ѧ���ķ��������������Ƕ�Ԫ�ػ�����֪ʶ�뷴Ӧԭ�����ۺϿ��飬ע���ھ���Ŀ����Ϣ����Һ����ԡ����ɰ�ɫ����������ȣ�ͬʱǰ����ϵ���з������

| ʵ�� | ���� | |
| A | ����ϡ���� | �Ƚ�Ũ��������ձ��У���������ˮ |
| B | ��ˮ���ռ�KMnO4�ֽ������O2 | ��Ϩ��ƾ��ƣ����Ƴ����� |
| C | Ũ������MnO2��Ӧ�Ʊ�����Cl2 | ���������ͨ��Ũ���ᣬ��ͨ������ʳ��ˮ |
| D | CCl4��ȡ��ˮ�е�I2 | �ȴӷ�Һ©���¿ڷų��л��㣬����Ͽڵ���ˮ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | �ӳɷ�Ӧ | B�� | �ۺϷ�Ӧ | C�� | ������Ӧ | D�� | ˮ�ⷴӦ |
| A�� | ����̼Ԫ�صĻ����ﲻһ������ | |
| B�� | ������������ĸ�̼ԭ�ӿ�����ͬһֱ���� | |
| C�� | ���³�ѹ�� ����̬ ����̬ | |
| D�� | �������κ�����¾�����ǿ�ᡢǿ���������Ӧ |
| A�� | ��ͭ����ϡ�����У�Cu+4H++2NO3-�TCu2++2NO2��+H2O | |
| B�� | ��Fe2��SO4��3��Һ�м���������ۣ�Fe3++Fe�T2Fe2+ | |
| C�� | ��Al2��SO4��3��Һ�м��������ˮ��Al3++3NH3•H2O�TAl��OH��3��+3NH4+ | |
| D�� | ��Na2SiO3��Һ�еμ�ϡ���Na2SiO3+3H+�TH2SiO3��+3Na+ |
| A�� | ������=A-n | B�� | ������=A-Z | C�� | ������=Z+n | D�� | ���������=n |


 ��
�� ����Ӧ������ȡ����Ӧ��
����Ӧ������ȡ����Ӧ��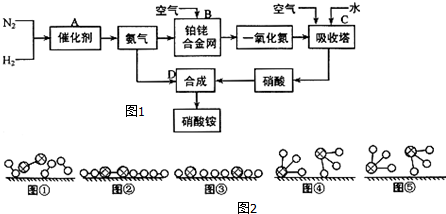
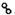 ��
�� ��
�� �ֱ��ʾH2��N2��NH3��ͼ�ݱ�ʾ���ɵ�NH3�뿪�������棬ͼ�ں͢ۺ���ֱ���N2��H2�������ڴ������桢�ڴ������棬N2��H2�л�ѧ�����ѣ�
�ֱ��ʾH2��N2��NH3��ͼ�ݱ�ʾ���ɵ�NH3�뿪�������棬ͼ�ں͢ۺ���ֱ���N2��H2�������ڴ������桢�ڴ������棬N2��H2�л�ѧ�����ѣ�