��Ŀ����
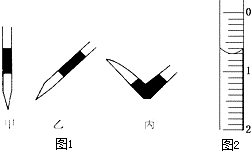 ʹ������к͵ζ����ⶨijδ֪���ʵ���Ũ�ȵ�ϡ���ᣮ
ʹ������к͵ζ����ⶨijδ֪���ʵ���Ũ�ȵ�ϡ���ᣮ��ʵ�鲽�裺
��1���ζ�ǰ����
�ٵζ��ܣ�
a����ȥ��ʽ�ζ��������ݵķ���Ӧ������ͼ1��ʾ�����е�
b����¼ʢװ0.1000mol/L NaOH��Һ�ļ�ʽ�ζ��ܵij�ʼ����ʱ��Һ��λ����ͼ2��ʾ�����ʱ�Ķ���Ϊ
����ƿ����
��2���ζ�
�ñ���NaOH��Һ�ζ������ϡ����ʱ�����ֲ����ζ��ܻ�����������ҡ��ƿ���۾�ע��
��ʵ���¼��
�ζ����� ʵ������/mL | 1 | 2 | 3 | 4 |
| V����Ʒ�� | 20.00 | 20.00 | 20.00 | 20.00 |
| V��NaOH�������ģ� | 15.80 | 15.00 | 15.02 | 14.98 |
��1������ϱ����ݣ����㱻��ϡ��������ʵ���Ũ����
��2���ڱ�ʵ���У����в���������������ȷ������ɲⶨ���ƫ�͵���
A����ƿˮϴ��δ����
B����ʽ�ζ���δ�ñ�NaOH��Һ��ϴ
C���ζ��յ����ʱ���Ӷ���
D����ƿ�ڵζ�ʱ����ҡ����������Һ�彦��
E�����Ʊ�Һ��NaOH�����л�������KOH����
F����ʽ�ζ��ܼ��첿�������ݣ��ζ�����ʧ��
���㣺�к͵ζ�
ר�⣺ʵ����
��������1���ٵζ���ʹ��ǰ�ȼ�©��
a�����ݼ�ʽ�ζ��ܵĽṹ�������ݵķ���������
b�����ݵζ��ܵĽṹ�뾫ȷ����������
��������Һ����ʽ�ζ�����ȡ��
��2������к͵ζ�ʱ���۾�Ҫע����ƿ����Һ����ɫ�仯��
��1���ȸ������ݵ���Ч�ԣ���ȥ��1�����ݣ�Ȼ�����2��3��4��ƽ������V��NaOH��Һ�������Ÿ��������NaOH��Ӧ���C�����ᣩ��
��2������c�����⣩=
��������������V��������Ӱ�죬�Դ��ж�Ũ�ȵ���
a�����ݼ�ʽ�ζ��ܵĽṹ�������ݵķ���������
b�����ݵζ��ܵĽṹ�뾫ȷ����������
��������Һ����ʽ�ζ�����ȡ��
��2������к͵ζ�ʱ���۾�Ҫע����ƿ����Һ����ɫ�仯��
��1���ȸ������ݵ���Ч�ԣ���ȥ��1�����ݣ�Ȼ�����2��3��4��ƽ������V��NaOH��Һ�������Ÿ��������NaOH��Ӧ���C�����ᣩ��
��2������c�����⣩=
| c(��ע)��V(��) |
| V(����) |
���
�⣺��1���ٴ��л����IJ���������ʵ��ǰҪ�����Ƿ�©ˮ�����ζ���ʹ��ǰ�ȼ�©��
�ʴ�Ϊ����©��
a����ʽ�ζ����������ݵķ������ѵζ��ܵĽ�ͷ�������������������ἷѹ������ʹ���첿�ֳ�����Һ���ų����ݣ�
�ʴ�Ϊ������
b���ζ���Һ��Ķ���0.70mL��
�ʴ�Ϊ��0.70��
��������Һ����ʽ�ζ�����ȡ����������ʽ�ζ�����ȡ���
�ʴ�Ϊ����ʽ�ζ��ܣ�
��2������к͵ζ�ʱ���۾�Ҫע����ƿ����Һ����ɫ�仯�����жϵζ��յ㣻
�ʴ�Ϊ����ƿ����Һ����ɫ�仯��
��1���������ݵ���Ч�ԣ���ȥ��1�����ݣ�Ȼ�����2��3��4��ƽ������V��NaOH��Һ��=15.00mL��
HCl+NaOH=NaCl+H2O
0.0200L��C�����ᣩ=0.015L��0.1000mol/L
��ã�C�����ᣩ=0.0750mol/L��
�ʴ�Ϊ��0.0750��
��2��A����ƿˮϴ��δ�������Һ�����ʵ������䣬��V��������Ӱ�죬����c�����⣩=
������c�����⣩���䣬��A����
B����ʽ�ζ���δ�ñ�NaOH��Һ��ϴ����Һ��Ũ��ƫС�����V������ƫ����c�����⣩=
������c�����⣩ƫ��B����
C���ζ��յ����ʱ���Ӷ��������V������ƫС������c�����⣩=
������c�����⣩ƫС����C��ȷ��
D����ƿ�ڵζ�ʱ����ҡ����������Һ�彦��������������ʵ�����С�����ĵı���Һ�������Ƶ����ƫС������c�����⣩=
������c�����⣩ƫС��D��ȷ��
E�����Ʊ�Һ��NaOH�����л�������KOH���壬���V������ƫ����c�����⣩=
������c�����⣩ƫ��E����
E����ʽ�ζ��ܼ��첿�������ݣ��ζ�����ʧ�����V������ƫ����c�����⣩=
������c�����⣩ƫ��F����
�ʴ�Ϊ��CD��
�ʴ�Ϊ����©��
a����ʽ�ζ����������ݵķ������ѵζ��ܵĽ�ͷ�������������������ἷѹ������ʹ���첿�ֳ�����Һ���ų����ݣ�
�ʴ�Ϊ������
b���ζ���Һ��Ķ���0.70mL��
�ʴ�Ϊ��0.70��
��������Һ����ʽ�ζ�����ȡ����������ʽ�ζ�����ȡ���
�ʴ�Ϊ����ʽ�ζ��ܣ�
��2������к͵ζ�ʱ���۾�Ҫע����ƿ����Һ����ɫ�仯�����жϵζ��յ㣻
�ʴ�Ϊ����ƿ����Һ����ɫ�仯��
��1���������ݵ���Ч�ԣ���ȥ��1�����ݣ�Ȼ�����2��3��4��ƽ������V��NaOH��Һ��=15.00mL��
HCl+NaOH=NaCl+H2O
0.0200L��C�����ᣩ=0.015L��0.1000mol/L
��ã�C�����ᣩ=0.0750mol/L��
�ʴ�Ϊ��0.0750��
��2��A����ƿˮϴ��δ�������Һ�����ʵ������䣬��V��������Ӱ�죬����c�����⣩=
| c(��ע)��V(��) |
| V(����) |
B����ʽ�ζ���δ�ñ�NaOH��Һ��ϴ����Һ��Ũ��ƫС�����V������ƫ����c�����⣩=
| c(��ע)��V(��) |
| V(����) |
C���ζ��յ����ʱ���Ӷ��������V������ƫС������c�����⣩=
| c(��ע)��V(��) |
| V(����) |
D����ƿ�ڵζ�ʱ����ҡ����������Һ�彦��������������ʵ�����С�����ĵı���Һ�������Ƶ����ƫС������c�����⣩=
| c(��ע)��V(��) |
| V(����) |
E�����Ʊ�Һ��NaOH�����л�������KOH���壬���V������ƫ����c�����⣩=
| c(��ע)��V(��) |
| V(����) |
E����ʽ�ζ��ܼ��첿�������ݣ��ζ�����ʧ�����V������ƫ����c�����⣩=
| c(��ע)��V(��) |
| V(����) |
�ʴ�Ϊ��CD��
������������Ҫ�������к͵ζ��������������Լ����㣬��Ŀ�Ѷ��еȣ������к͵ζ���ԭ���ǽ���ؼ��������ڿ���ѧ����ʵ�����������ݴ���������

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
��Mg��MgO��Mg��OH��2��MgCO3��MgSO4��BaCl2��Һ������Ϊԭ�ϣ�ֱ����ȡM���ж��ַ������������ø��ֽⷴӦ�ģ�������
| A������ | B������ | C������ | D������ |
��������ʢ�������������е������ǣ�������
| A��Ũ���� | B��Ũ���� |
| C������ͭ��Һ | D��ϡ���� |
50mL 18.4mol/L������������ͭƬ���ȷ�Ӧ������ԭ����������ʵ����ǣ�������
| A��0.92mol |
| B������0.46mol ��0.92mol |
| C��0.46mol |
| D����0.46mol |
�ڼ�����Һ���ܴ�����������ҺΪ��ɫ�����������ǣ�������
| A��K+��MnO4-��Na+��Cl- |
| B��K+��Na+��NO3-��CO32- |
| C��Na+��H+��NO3-��SO42- |
| D��Al3+��Na+��Cl-��SO42- |
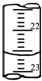 ijѧ����0.2000mol?L-1�ı�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ��������£�
ijѧ����0.2000mol?L-1�ı�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ��������£�
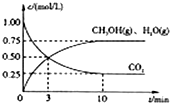 �������������ЧӦ����Դ��ȱ����������ӣ���ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2�������˸������ձ����ӣ�
�������������ЧӦ����Դ��ȱ����������ӣ���ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2�������˸������ձ����ӣ�