��Ŀ����
10�����������Ի���Ӱ��ܴ�����SO2����ɿ�����Ⱦ����Ҫԭ�������Ƽ�ѭ�����ɳ�ȥSO2����1�������£�����Һ����SO2�Ĺ����У�pH��n��SO32������n��HSO3�����仯��ϵ���±���
| n��SO32������n��HSO3���� | 91��9 | 1��1 | 1��91 |
| pH | 8.2 | 7.2 | 6.2 |
| c��H+��/mol•L��1 | 6.3��10��9 | 6.3��10��8 | 6.3��10��7 |
����NaHSO3��Һ������Ũ�ȹ�ϵ����ȷ����A������ţ���
A��c��Na+��=2c��SO32����+c��HSO3����
B��c��Na+����c��HSO3������c��H+����c��SO32������c��OH����
C��c��H2SO3��+c��H+��=c��SO32����+c��OH����
D��c��Na+��+c��H+��=2c��SO32-��+c��HSO3����+c��OH����
�ۼ���������HSO3��?H++SO32���ĵ���ƽ�ⳣ��K=6.3��10-8������2λ��Ч���֣���
��2��NaHSO3��Һ�ڲ�ͬ���¶��¾��ɱ�������KIO3��������NaHSO3��ȫ���ļ���I2��������Ũ�Ⱦ�Ϊ0.02mol•L-1 ��NaHSO3��Һ�����������ۣ�10.0mL�� KIO3��������������Һ40.0mL��ϣ���¼��Һ����ʱ�䣬ʵ������ͼ��

����ͼ��֪����Һ������ʱ�����¶ȵı仯������40��֮ǰ���¶�Խ�ߣ���Һ��������ʱ��Խ�̣�40��֮���¶�Խ�ߣ���Һ��������ʱ��Խ����
40��֮���۲�����������ʵ���ָʾ��������ˡ������ˡ�����ԭ���ǣ��¶ȸ���40��ʱ��������۵���ɫ��Ӧ�����Ƚ��ͣ�����ۻ��������
��b���c���Ӧ�ķ�Ӧ���ʵĴ�С��ϵ�Ǧ� ��b������ ��c�������������������������
���� ��1�����ɱ����е����ݿ�֪��HSO3-Խ�࣬����Խǿ����������������ӣ�
����NaHSO3��Һ�У�����̶ȴ���ˮ��̶ȣ���Һ�����ԣ�
A���������غ������
B����NaHSO3��Һ�У�����̶ȴ���ˮ��̶ȣ���Һ�����ԣ�
C�����������غ������
D�����ݵ���غ������
�۸���HSO3-?H++SO32-����֪Ka=$\frac{c��S{{O}_{3}}^{2-}����c��{H}^{+}��}{c��HS{{O}_{3}}^{-}��}$������n��SO32-����n��HSO3-��=1��1��c��H+��=6.3��10-8���㣻
��2������ͼ��֪��40��֮ǰ���¶ȸ߷�Ӧ���ʼӿ죬40��֮���¶ȸߣ���ɫʱ��Խ�����¶�̫�ߣ�������۵���ɫ��Ӧ�����Ƚ��ͣ�����ۻ��������
���¶�Խ�߷�Ӧ����Խ�죮
��� �⣺��1�����ɱ����е����ݿ�֪��HSO3-Խ�࣬����Խǿ����������������ӣ����뷽��ʽΪHSO3-?H++SO32-����NaHSO3��Һ��HSO3-����HSO3-?H++SO32-��HSO3-+H2O?H2SO3+OH-����ƽ�⣬HSO3-�ĵ���̶ȴ�����ˮ��̶ȣ�����Һ�����ԣ�
�ʴ�Ϊ�����NaHSO3��Һ��HSO3-����HSO3-?H++SO32-��HSO3-+H2O?H2SO3+OH-����ƽ�⣬HSO3-�ĵ���̶ȴ�����ˮ��̶ȣ�����Һ�����ԣ�
����NaHSO3��Һ�У�����̶ȴ���ˮ��̶ȣ���Һ�����ԣ�
A���������غ��֪��c��Na+��=c��SO32-��+c��HSO3-��+c��H2SO3������A����
B����NaHSO3��Һ�У�����̶ȴ���ˮ��̶ȣ���Һ�����ԣ�������Ũ��Ϊc��Na+����c��HSO3-����c��H+����c��SO32-����c��OH-������B��ȷ��
C�����������غ㣬c��H2SO3��+c��H+���Tc��SO32-��+c��OH-������C��ȷ��
D�����ݵ���غ�c��Na+��+c��H+��=2c��SO32-��+c����HSO3-��+c��OH-������D��ȷ��
�ʴ�Ϊ��A��
�۸���HSO3-?H++SO32-����֪Ka=$\frac{c��S{{O}_{3}}^{2-}����c��{H}^{+}��}{c��HS{{O}_{3}}^{-}��}$����֪n��SO32-����n��HSO3-��=1��1��c��H+��=6.3��10-8����Ka=$\frac{1��6��{3}^{-8}}{1}$=6.3��10-8��
�ʴ�Ϊ��6.3��10-8��
��2���ٴ�ͼ���п��Կ�����40����ǰ���¶�Խ�ߣ���Ӧ�ٶ�Խ�죬40����¶�Խ�ߣ���ɫʱ��Խ������ӦԽ������55�棬δ������˵��û������I2���¶�̫�ߣ�������۵���ɫ��Ӧ�����Ƚ��ͣ�����ۻ������������40��֮���۲���������ʵ���ָʾ����
�ʴ�Ϊ��40��֮ǰ���¶�Խ�ߣ���Һ��������ʱ��Խ�̣�40��֮���¶�Խ�ߣ���Һ��������ʱ��Խ�����¶ȸ���40��ʱ��������۵���ɫ��Ӧ�����Ƚ��ͣ�����ۻ��������
���¶�Խ�߷�Ӧ����Խ�죬c����¶ȸߣ�����c��ķ�Ӧ���ʴ��� ��b������ ��c����
�ʴ�Ϊ������
���� ���⿼����������ʵĵ�����ε�ˮ�⡢��ѧ��Ӧ���ʵ�Ӱ�����صȣ��ۺ��Խ�ǿ���漰��������ʵĵ��롢����Ũ�ȴ�С�ıȽϡ���ѧ��Ӧ���ʵ�Ӱ�����ص�֪ʶ�㣬��Ŀ�ѶȽϴ���Ҫע����DZȽ���Һ�и�������Ũ����Դ�СʱҪ��ϵ���غ�������غ�������

| A�� | ��12C��Ϊͬ���������14C��������������ļ��� | |
| B�� | �Ӻ�ˮ����ȡ���ʲ�һ����Ҫͨ����ѧ��Ӧʵ�� | |
| C�� | Ϊ�����й©�¹ʵ�Σ����Ӧ�ƹ�ȼú���磬ͣ���˵�վ | |
| D�� | ���ۡ���֬�������ʵȶ�����Ȼ�߷��ӻ����� |
 NH4HSO4��ˮ��Һ�еĵ��뷽��ʽΪ��NH4HSO4=NH4++H++SO42-������100mL 0.1mol/LNH4HSO4��Һ�еμ�0.1mol/LNaOH��Һ���õ�����ҺpH��NaOH��Һ����Ĺ�ϵ������ͼ��ʾ������˵���в���ȷ���ǣ�������
NH4HSO4��ˮ��Һ�еĵ��뷽��ʽΪ��NH4HSO4=NH4++H++SO42-������100mL 0.1mol/LNH4HSO4��Һ�еμ�0.1mol/LNaOH��Һ���õ�����ҺpH��NaOH��Һ����Ĺ�ϵ������ͼ��ʾ������˵���в���ȷ���ǣ�������| A�� | a�����Һ�У�c��SO42-����c��NH4+����c��H+����c��Na+����c��OH-�� | |
| B�� | b�����Һ�У�c��Na+��=c��SO42-����c��H+����c��NH4+����c��OH-�� | |
| C�� | c�����Һ�У�c��Na+����c��SO42-����c��NH4+����c��OH-��=c��H+�� | |
| D�� | d��e���Ӧ��Һ�У�ˮ����̶ȴ�С��ϵ��d��e |
| A�� | M���⻯���ˮ��Һ����W��������Һ����Ӧ�õ�W���⻯�˵���ǽ�����M��W | |
| B�� | ԭ�Ӱ뾶�Ĵ�С˳��r��W����r��Z����r��Y�� | |
| C�� | Y��Z�γɵĻ���������Ӧ�������ܺ��κ��ᷴӦ | |
| D�� | X��Y��W��M�����γ�ԭ�Ӹ�����Ϊ1��1�ķ��� |
| A�� | ���ʵķе㣺Z��X��Y | |
| B�� | ���ʵ������ԣ�W��Z��Y��X | |
| C�� | ��̬�⻯����ȶ��ԣ�W��X��Y��Z | |
| D�� | W���ʿ��Խ�X��������Һ���û����� |
| A�� | ������Ϊ18����ԭ�ӣ�${\;}_{16}^{34}$S | |
| B�� | �����ӵĵ���ʽ��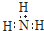 | |
| C�� | �������ƵĽṹ��ʽ��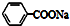 | |
| D�� | H2SO3�ĵ��뷽��ʽ��H2SO3?2H++SO32- |
 ��N2��H2Ϊ��Ӧ�����A��ϡ����Ϊ�������Һ�����Ƴ��̵ܹ�������ȼ�ϵ�أ�ԭ����ͼ��ʾ������˵������ȷ���ǣ�������
��N2��H2Ϊ��Ӧ�����A��ϡ����Ϊ�������Һ�����Ƴ��̵ܹ�������ȼ�ϵ�أ�ԭ����ͼ��ʾ������˵������ȷ���ǣ�������| A�� | b�缫Ϊ����������������Ӧ | |
| B�� | a�缫�����ķ�ӦΪN2+8H++6e-=2NH4+ | |
| C�� | A��Һ����������ΪNH4Cl | |
| D�� | ����Ӧ����1molN2ʱ�������ĵ�H2Ϊ67.2L |
| A�� | 4��10 | B�� | 10��4 | C�� | 4��1 | D�� | 1��4 |
��1������ͭ��Һ���Լӿ������������ʵ�ԭ����CuSO4��Zn��Ӧ������Cu��Zn�γ�ͭпԭ��أ��ӿ����������������ʣ�
��2��Ҫ�ӿ�����ʵ����������������ʣ����ɲ�ȡ�Ĵ�ʩ�����߷�Ӧ�¶ȡ��ʵ����������Ũ�ȣ������֣���
��3��Ϊ�˽�һ���о�����ͭ�����������������ʵ�Ӱ�죬��ͬѧ���������һϵ��ʵ�飮�����������Ļ����Һ�ֱ���뵽6��ʢ�й���Zn���ķ�Ӧƿ�У��ռ����������壬��¼�����ͬ�������������ʱ�䣮
| ʵ�� �����Һ | A | B | C | D | E | F |
| 4mol/L H2SO4/mL | 30 | V1 | V2 | V3 | V4 | V5 |
| ����CuSO4��Һ/mL | 0 | 0.5 | 2.5 | 5 | V6 | 20 |
| H2O/mL | V7 | V8 | V9 | V10 | 10 | 0 |
�ڸ�ͬѧ���ó��Ľ���Ϊ������������CuSO4��Һʱ���������������ʻ�����ߣ����������CuSO4��Һ����һ����ʱ���������������ʷ������½���������������������½�����Ҫԭ����һ����������ͭ�����ɵĵ���ͭ�������п�ı��棬������п����Һ�ĽӴ������