��Ŀ����
15�� ijС����CoCl2•6H2O��NH4Cl��H2O2��Ũ��ˮΪԭ�ϣ��ڻ���̿���£��ϳ��˳Ȼ�ɫ����X��Ϊȷ������ɣ���������ʵ�飮
ijС����CoCl2•6H2O��NH4Cl��H2O2��Ũ��ˮΪԭ�ϣ��ڻ���̿���£��ϳ��˳Ȼ�ɫ����X��Ϊȷ������ɣ���������ʵ�飮�ٰ��IJⶨ����ȷ��ȡw g X��������ˮ�ܽ⣬ע����ͼ��ʾ������ƿ�У�Ȼ����μ�������10%NaOH��Һ��ͨ��ˮ����������ƷҺ�еİ�ȫ����������V1 mL c1 mol•L-1���������Һ���գ�����������ȡ�½���ƿ����c2 mol•L-1 NaOH����Һ�ζ���ʣ��HCl�����յ�ʱ����V2 mL NaOH��Һ��
���ȵIJⶨ
�ش��������⣺
��1��װ���а�ȫ�ܵ�����ԭ����ʹAƿ��ѹǿ�ȶ���
��2����NaOH����Һ�ζ���ʣ��HClʱ��Ӧʹ�ü�ʽ�ζ��ܣ���ʹ�õ�ָʾ��Ϊ��̪����ȣ�
��3����Ʒ�а���������������ʽΪ$\frac{��C{\;}_{1}V{\;}_{1}-C{\;}_{2}V{\;}_{2}����10{\;}^{-3}��17}{w}$��100%��
��4���ⶨ��ǰӦ�ö�װ�ý��������Լ�飬�������Բ��òⶨ�����ƫ�ͣ��ƫ�ߡ���ƫ�͡�����
��5���ȵIJⶨ����Ī������Ī������һ�ֳ����ζ������ñ���������Һ�ζ������вⶨ��Һ��Cl-��Ũ�ȣ���֪��
| ���� ���� | AgCl | AgBr | AgCN | Ag2CrO4 | AgSCN |
| ��ɫ | �� | dz�� | �� | ש�� | �� |
| �ܽ�ȣ�mol•L-1�� | 1.3410-6 | 7.110-7 | 1.110-8 | 6.510-5 | 1.010-6 |
A��NaCl B��BaBr2 C��Na2CrO4 D��KSCN
�ڵζ��յ�������ǵ������һ�α���Һ������ש��ɫ��������30s ����ԭ��
���� ��1��ͨ��2��Һ�����A��ѹǿ��
��2����ֻ��ʢ���ڼ�ʽ�ζ����У�������Һֻ��ʢ������ʽ�ζ����У�NaOH��Һ��������Һǡ�÷�Ӧ������ԣ�����ѡ�����Ի���Ա�ɫ��Χ�ڵ�ָʾ����
��3�����ݰ���������ᷴӦ֮��Ĺ�ϵʽ���㰱�����������ٸ�������������ʽ���㰱����������
��4���������Բ��ã����°�������ƫ�ͣ�
��5����ֻ�е��ζ����ͱ��ζ������������ܽ�ȱȵζ�����ָʾ������������ܽ��Сʱ����Ӧ�ܽ��У�
��Na2CrO4Ϊָʾ����Ag2CrO4Ϊש��ɫ���ñ��������ζ�����Һ���ζ��յ�������ǵ������һ�α���Һ������ש��ɫ��������30s ����ԭ��
��� �⣺��1����������ƿ��ѹǿ������С�����������Σ�գ�������A�ڵ�����Һ�����ߣ�������ѹ��������С��������ͨ�����ܽ�����ƿ��Ҳ������ɵ�������ȫ���õ�ԭ����ʹA��ѹǿ�ȶ���
�ʴ�Ϊ��ʹAƿ��ѹǿ�ȶ���
��2����ֻ��ʢ���ڼ�ʽ�ζ����У�������Һֻ��ʢ������ʽ�ζ����У�������NaOH����Һȷ����ʣ��HClʱ��Ӧʹ�ü�ʽ�ζ���ʢ��NaOH��Һ��NaOH��Һ��������Һǡ�÷�Ӧ������ԣ�����ѡ�����Ի���Ա�ɫ��Χ�ڵ�ָʾ��������Ϊ���Ա�ɫָʾ������̪Ϊ���Ա�ɫָʾ�������Կ���ѡȡ���Ȼ��̪��ָʾ����
�ʴ�Ϊ�����̪����ȣ�
��3���백����Ӧ��n��HCl��=V1��10-3L��C1mol•L-1-C2mol•L-1 ��V2��10-3L=��C1V1-C2V2����10-3mol�����ݰ�����HCl�Ĺ�ϵʽ֪��n��NH3��=n��HCl��=��C1V1-C2V2����10-3mol��������������=$\frac{��C{\;}_{1}V{\;}_{1}-C{\;}_{2}V{\;}_{2}����10{\;}^{-3}mol��17g/mol}{wg}$��100%=$\frac{��C{\;}_{1}V{\;}_{1}-C{\;}_{2}V{\;}_{2}����10{\;}^{-3}��17}{w}$��100%��
�ʴ�Ϊ��$\frac{��C{\;}_{1}V{\;}_{1}-C{\;}_{2}V{\;}_{2}����10{\;}^{-3}��17}{w}$��100%��
��4���������Բ��ã����²��ְ���й©����������������ƫ�ͣ��ʴ�Ϊ��ƫ�ͣ�
��5��������AgNO3ȥ�ζ�Cl-��Ũ�ȣ���ѡ�õĵζ�ָʾ���������ӷ�Ӧ��������ʵ��ܽ��Ӧ��AgCl�����������ԣ������ݿ���ӦΪNa2CrO4����ѡ��C��
�ڵζ��յ�������ǵ������һ����������Һʱ����Һ�г���ש��ɫ��������������30s ����ԭ��
�ʴ�Ϊ���������һ�α���Һ������ש��ɫ��������30s ����ԭ��
���� ���⿼�������ʺ����IJⶨ���漰��������ܽ�ƽ�⡢������ԭ��Ӧ�����ʺ����IJⶨ��֪ʶ�㣬��ȷʵ��ԭ���ǽⱾ��ؼ���֪��ָʾ����ѡȡ��������Ŀ�Ѷ��еȣ�

 ������ȫ�̼����ĩ���100��ϵ�д�
������ȫ�̼����ĩ���100��ϵ�д�| A�� | ����Ӧ�Ƿ��ȷ�Ӧ�Ŀ��淴Ӧ�������¶�v��������v���棩 | |
| B�� | �����¶Ȼ�ʹ�ô�����ͨ�����ӻ���Ӱٷ�����ʹ��Ӧ���ʼӿ� | |
| C�� | ��������ԭ��ֻʹ���ڿ��淴Ӧ����ʹ�����ܽ��������ʵĵ��� | |
| D�� | ��G=��H-T��S�ʺ����������Ļ�ѧ��Ӧ���еķ����о� |
��1���ζ�Ӧ��ѡ�÷�̪��ָʾ��
��2�����¶���CH3COOH�ĵ��볣��Ka=2��10-5��
��3�������ʵ��Ũ��Ϊ0.1006mol/L��������λ��Ч���֣�
��4�����������������ⶨ���ƫ�ߵ���AC��
A����ʽ�ζ���δ�ñ���Һ��ϴ
B����ƿδ�ô���Һ��ϴ
C���ζ�ǰ�ζ��ܼ�������һ���ݣ��ζ���������ʧ��
D���ζ�ǰ���ζ����е���ҺҺ����͵��ڡ�0��������
��5���ζ��ķ���������к͵ζ��������ζ�����ϵζ���������ԭ�ζ��ȣ������ζ����õ�ָʾ����������һ�ֳ���������֪һЩ���ε���ɫ��Ksp��20�棩���£��ⶨˮ�����Ȼ���ĺ��������ñ���������Һ���еζ���
| ��ѧʽ | AgCl | AgBr | AgI | Ag2S | Ag2CrO4 |
| ��ɫ | ��ɫ | dz��ɫ | ��ɫ | ��ɫ | ��ɫ |
| Ksp | 1.8��10-10 | 5.0��10-13 | 8.3��10-17 | 2.0��10-48 | 1.8��10-10 |
A��KBr B��KI C��K2S D��K2CrO4
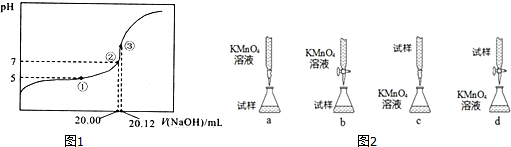
��6�������������£���KMnO4����Һ����������ԭ�ζ������ԲⶨFe2+�ĺ�������Ӧ�����ӷ���ʽ�ǣ�Fe2++MnO4-+H+--Fe3++Mn2++H2O��δ��ƽ��ͼ2���ֵζ���ʽ���гֲ�����ȥ��������Ϊ���������b������ĸ��ţ����жϵζ��յ�����ݵ������һ��KMnO4��Һǡ�����ػ�ɫ����ɫ���Ұ�����ڲ���ɫ��
��1��ʵ�鲽��Ϊ������������ƽȷ����4.1g�ռ���Ʒ��
�ڽ���Ʒ���250ml����Һ��
����ȡ10.00ml����Һ��ע����ƿ�У�
������ƿ�е���2��3�η�̪����0.2010mol/L�ı�����ζ������ռ���Һ��
��2���������������
| �ζ����� | ����Һ���/ml | ���������/ml | |
| �ζ�ǰ����/ml | �ζ������/ml | ||
| ��һ�� | 10.00 | 0.20 | 22.90 |
| �ڶ��� | 10.00 | 0.50 | 20.40 |
| ������ | 10.00 | 4.00 | 24.10 |
| ���Ĵ� | 10.00 | 0.00 | 20.00 |
| A�� | ʹ�ô����ܼӿ췴Ӧ���ʣ�ʹN2��ȫת��ΪNH3 | |
| B�� | ��N2��H2��NH3Ũ�����ʱ����Ӧ�ﵽ��ѧƽ��״̬ | |
| C�� | һ��ʱ���N2��H2��NH3Ũ�Ȳ��ٸı�ʱ����Ӧ��ת������� | |
| D�� | ��ƽ��������¶ȣ���Ӧ���ʼ��� |
 ijͬѧ��0.10mol/L��HCl��Һ�ⶨδ֪Ũ�ȵ�NaOH��Һ����ʵ��������£�
ijͬѧ��0.10mol/L��HCl��Һ�ⶨδ֪Ũ�ȵ�NaOH��Һ����ʵ��������£�
 ��
��