��Ŀ����
11����������ƣ�Na2S2O3���׳ơ����������ߡ����մ���ˮ��Һ���������ԣ���������Һ�в��ȶ������н�ǿ�Ļ�ԭ�ԣ�����֯��Ư������ȼ������������еĻ�ԭ������������ƿ����������ƺ����ͨ�����Ϸ�Ӧ�Ƶã�װ����ͼ��a����ʾ���й����ʵ��ܽ��������ͼ��b����ʾ��
��1��Na2S2O3•5H2O���Ʊ���
����1����ͼ��a�����Ӻ�װ�ú��A��Cװ�������ԵIJ����ǹر�K2��K1����D�м�ˮ��û����ĩ�ˣ�����ë����˫����ס��ƿ��D�е���������ð������ȴ���γ�һ��ˮ����˵�����������ã�
����2������ҩƷ����K1���ر�K2�����ȣ�װ��B��D�е�ҩƷ��ѡ�õ���A��C��D�����ţ���
A��NaOH��Һ B��ŨH2SO4 C������KMnO4��Һ D������NaHCO3��Һ
����3��C�л��Һ��������������Ӧһ��ʱ�����۵������٣���C����Һ��7��pH��10ʱ����K2���ر�K1��ֹͣ���ȣ�C����ҺҪ����pH��ԭ���������������������Һ�в��ȶ���
����4������C�еĻ��Һ������Һ��������Ũ�������ȹ��ˣ��ٽ���Һ��ȴ�ᾧ�����½ᾧ�������ˡ�ϴ�ӡ���ɣ��õ���Ʒ������ȹ��˵�ԭ���Ƿ�ֹ��������ƽᾧ������
��2��Na2S2O3���ʵļ��飺��������������ˮ�еμ�����Na2S2O3��Һ����ˮ��ɫ��dz����鷴Ӧ����Һ�к���SO42-��д���÷�Ӧ�Ļ�ѧ����ʽS2O32-+4Cl2+5H2O=2SO42-+10H++8Cl-��
��3������Na2S2O3��Һ�ⶨ��ˮ��Ba2+Ũ�ȣ��������£�ȡ25.00mL��ˮ��������K2Cr2O7������Һ����BaCrO4���������������ܽ��������ʱCrO42-ȫ��ת��ΪCr2O2-7���ټӹ���KI��Һ����ַ�Ӧ����0.010mol•L-1��Na2S2O3��Һ���еζ����õ�����Һ��ָʾ��������Ӧ��ȫʱ������Na2S2O3��Һ18.00mL���йط�Ӧ�����ӷ���ʽΪ��Cr2O72-+6I-+14H+�T3I2+2Cr3++7H2O��I2+2S2O32-�TS4O62-+2I-����÷�ˮ��Ba2+�����ʵ���Ũ��Ϊ0.0024mol/L��
���� ��1������1���������������������ʣ�����װ�������ԣ�
����2��װ��B��D�������ǽ���β����������ֹβ���ж���������Ⱦ������
����3�������������������Һ�в��ȶ���Ӧ������ҺΪ�����ԣ���������ҺpH�ӽ���С��7��
����4������Һ�л�þ��壬��Ҫ����Ũ�������ȹ��ˣ��ٽ���Һ��ȴ�ᾧ�����ˡ�ϴ�ӡ���ɣ��õ���Ʒ�������ܽ������ͼ��֪���ȹ��˵�ԭ���Ƿ�ֹ��������ƽᾧ������
��2������Ŀ��Ϣ��֪��Na2S2O3����ˮ������Ӧ����Na2SO4��H2SO4����������ԭΪHCl��
��3���������֪��BaCrO4�������ܽ�ת��ΪCr2O2-7����Ԫ���غ㼰��֪����ʽ�ɵù�ϵʽ��2Ba2+��2BaCrO4��Cr2O2-7��3I2��6Na2S2O3��������ĵ�Na2S2O3���ù�ϵʽ������Һ��n��Ba2+������������c��Ba2+����
��� �⣺��1������1���������������������ʣ�����װ�������ԣ��������Ϊ���ر�K2��K1����D�м�ˮ��û����ĩ�ˣ�����ë����˫����ס��ƿ��D�е���������ð������ȴ���γ�1��ˮ����˵�����������ã�
�ʴ�Ϊ���ر�K2��K1����D�м�ˮ��û����ĩ�ˣ�����ë����˫����ס��ƿ��D�е���������ð������ȴ���γ�һ��ˮ����˵�����������ã�
����2��װ��B��D�������ǽ���β����������ֹβ���ж���������Ⱦ����������������л�ԭ�ԣ����������Ը��������Һ�������գ�����������������������Һ��̼��������Һ��Ӧ�����գ�
�ʴ�Ϊ��ACD��
����3�������������������Һ�в��ȶ���Ӧ������ҺΪ�����ԣ����Կ�����ҺpH�ӽ���С��7��
�ʴ�Ϊ�������������������Һ�в��ȶ���
����4������Һ�л�þ��壬��Ҫ����Ũ�������ȹ��ˣ��ٽ���Һ��ȴ�ᾧ�����ˡ�ϴ�ӡ���ɣ��õ���Ʒ�������ܽ������ͼ��֪̼�������¶������ܽ�Ƚ��ʹӶ���������������Ƶ��ܽ�����¶����߶������ȹ��˿ɳ���̼���ƾ��壬�����ȴ���ٹ��˻ᵼ����������ƽᾧ������
�ʴ�Ϊ����ȴ�ᾧ�����½ᾧ������ֹ��������ƽᾧ������
��2������Ŀ��Ϣ��֪��Na2S2O3����ˮ������Ӧ����Na2SO4��H2SO4����������ԭΪHCl����Ӧ�����ӷ���ʽΪ��S2O32-+4Cl2+5H2O=2SO42-+10H++8Cl-��
�ʴ�Ϊ��NaS2O32-+4Cl2+5H2O=2SO42-+10H++8Cl-��
��3���������֪��BaCrO4�������ܽ�ת��ΪCr2O2-7����Ԫ���غ㼰��֪����ʽ�ɵù�ϵʽ��2Ba2+��2BaCrO4��Cr2O2-7��3I2��6Na2S2O3�����ĵ�Na2S2O3Ϊ0.018L��0.01mol/L����n��Ba2+��=0.018L��0.01mol/L��$\frac{1}{3}$=0.00006mol������Һ��c��Ba2+��=$\frac{0.00006mol}{0.025L}$=0.0024mol/L��
�ʴ�Ϊ��0.0024mol/L��
���� ���⿼��ʵ���Ʊ�������ƣ��漰�����Լ��顢��ʵ��װ�ü�����ķ������ۡ����ʵķ����ᴿ��������ԭ��Ӧ�ζ�����3����ע�����ù�ϵʽ���м��㣬�Ѷ��еȣ�

| A�� | �٣��ڣ��� | B�� | �ۣ��٣��� | C�� | �ۣ��ڣ��� | D�� | �ڣ��٣��� |
| A�� | ��������ѹǿ | B�� | �������������ʵ��� | ||
| C�� | ��������ƽ����Է������� | D�� | v��B��=3V��D�� |
| A�� | ��λʱ��������nmolA2��ͬʱ����2n molAB | |
| B�� | AB���������ʵ���A2���������� | |
| C�� | �����ڣ�3������AB��A2��B2���� | |
| D�� | �����и���ֵ������������ʱ��仯 |
| A�� | �ؽ�����ũҩ�Ȼ����ˮ����Ⱦ | |
| B�� | װ�β����еļ�ȩ�����Ȼ���ɾ�����Ⱦ | |
| C�� | CO�ᵼ��������γ� | |
| D�� | CO2�Ĵ����ŷŻ�Ӿ�����ЧӦ |
 +��2n-1��H2O
+��2n-1��H2O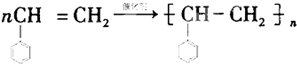
 ��NaOH�ķ�Ӧ
��NaOH�ķ�Ӧ

 _��
_�� ��
��