��Ŀ����
2��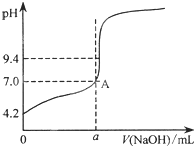 �����ڱ���������ر���Һ���ⶨNaOH��Һ��Ũ�ȣ�������������Һ���ζ��ڱ���������Һʱ�����в�����
�����ڱ���������ر���Һ���ⶨNaOH��Һ��Ũ�ȣ�������������Һ���ζ��ڱ���������Һʱ�����в�����������Һ�м���1��2��ָʾ�� ��ȡ20mL����Һ������ƿ��
��������������Һ�ζ����յ� ���ظ����ϲ���
������ƽ��ȷ��ȡ5.105g�ڱ���������أ���Է�������Ϊ204.2���������250mL����Һ�����pHԼΪ4.2����
����ʵ�����ݼ����������Ƶ����ʵ���Ũ�ȣ�
��1�����ϸ����У���ȷ�ģ�����ţ�����˳���Ǣݢڢ٢ۢܢޣ���������ʹ�õ���������ƿ�⣬����Ҫʹ�õ���������ʽ�ζ��ܣ�ѡ��ָʾ���ǣ���̪
��2���ζ�������¼NaOH���ն������ظ��ζ����Σ����ݼ�¼���±���
| �ζ����� ʵ������ | 1 | 2 | 3 | 4 |
| V����Ʒ��/mL | 20.00 | 20.00 | 20.00 | 20.00 |
| V��NaOH��/mL���������� | 0.10 | 0.30 | 0.00 | 0.20 |
| V��NaOH��/mL���ն����� | 20.08 | 20.30 | 20.80 | 20.22 |
| V��NaOH��/mL�����ģ� | 19.98 | 20.00 | 20.80 | 20.02 |
V��NaOH��=$\frac{19.98+20.00+20.80+20.02}{4}$20.20mL�����ļ�������������Dz���������3�����ݺ������������ϴ�Ӧ����
ͨ��������õ�4�εζ���������ҺpH������� ��������Һ����ı仯������ͼ��ʾ����a��20.02�����������������=����
��3����������ڹ۲�ζ��ܵ���ʼ����ʱ��Ҫʹ�ζ��ܵļ��첿�ֳ�����Һ������ζ����ڲ������ݣ��������ݵIJ������ٷ�Һ��
��4���ζ�ǰ��������ˮϴ����ʽ�ζ��ܣ�Ȼ��Ӵ��ⶨ������������Һ�ζ����˲�����ʵ����ƫС���ƫ����ƫС������Ӱ�족��
���� ��1�������к͵ζ��м�©��ϴ�ӡ���ϴ��װҺ��ȡ����Һ���ζ��Ȳ��������жϣ��ζ�ʱ���õζ��ܴ���Һȡ20mL����Һ������ƿ�У����ݵζ��յ��pHҪ��ָʾ���ı�ɫ��Χ֮��ȷ��ָʾ����
��2����3�����ݺ������������ϴ�����������Һ�����ȡ����3��ƽ��ֵ��
��3���ڱ���������ر���Һʢ������ʽ�ζ����У����ٷ�Һ���ɸ�����ʽ�ζ����е����ݣ�
��4���ζ�ǰ��������ˮϴ����ʽ�ζ��ܣ�Ȼ��Ӵ��ⶨ������������Һ�ζ�������Һ��ϡ�ͣ�
��� �⣺��1���к͵ζ����ռ�©��ϴ�ӡ���ϴ��װҺ��ȡ����Һ���ζ���˳������������˳��Ϊ�ݢڢ٢ۢܢޣ��ζ�ʱ������ʽ�ζ��ܴ���Һȡ20mL�ڱ���������ط�����ƿ�У��ڱ����������Ϊ���ᣬ�յ�ʱ��Һ��pHԼΪ9.1���ζ��յ��pHҪ��ָʾ���ı�ɫ��Χ֮�ڣ�����ѡ�÷�̪��ָʾ����
�ʴ𰸣��ݢڢ٢ۢܢޣ���ʽ�ζ��ܣ���̪��
��2����3�����ݺ������������ϴ�����������Һ�����ȡ����3��ƽ��ֵΪ��$\frac{17.98+20.00+20.02}{3}$mL=20.00mL��
�ʴ�Ϊ������������3�����ݺ������������ϴ�Ӧ���ã�����
��3���ڱ���������ر���Һʢ������ʽ�ζ����У�����ζ����ڲ������ݣ��������ݵIJ������ٷ�Һ���ʴ�Ϊ�����ٷ�Һ��
��4���ζ�ǰ��������ˮϴ����ʽ�ζ��ܣ�Ȼ��Ӵ��ⶨ������������Һ�ζ�������Һ��ϡ�ͣ�����Һ��Ũ��ƫС���ʴ�Ϊ��ƫС��
���� ���⿼�����к͵ζ��������������Լ����㣬�ѶȲ��������к͵ζ���ԭ���ǽ���ؼ���

 ��������ϵ�д�
��������ϵ�д�| A�� | �����ǵķ���ʽ��C6H12O6 | |
| B�� | �������Ƕ��ǻ�ȩ���������ȩ�Ͷ�Ԫ�������� | |
| C�� | �����������ȩ�� | |
| D�� | �����ǿ���ͨ����ɫֲ�������úϳ� |
| A�� | �ζ�ǰ���ζ��ܼ��첿�������ݣ��ζ���������ʧ | |
| B�� | ��ƿմ������ˮ | |
| C�� | �Լ�����ָʾ�� | |
| D�� | �ζ�ǰ���ӵζ��ܶ������ζ���ƽ�ӿ̶ȶ��� |
| A�� | ����AgCl��������Һ�м���NaC1���壬c��Ag+����С | |
| B�� | ������100mL pH=1.3��Ba��OH��2��Һ��OH-�����ʵ���Ϊ0.02 mol | |
| C�� | ϡ��0.1 mol/L��NH3•H2O��Һ����Һ����������Ũ�Ⱦ���С | |
| D�� | ��Һ��ˮ�����c��H+����ˮ�������c��OH-���ij˻�һ������10-14 |
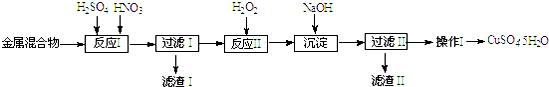
��֪������������������������ʽ����ʱ��Һ��pH���±���
| ������ | Fe3+ | Fe2+ | Cu2+ |
| ��ʼ���� | 1.5 | 6.4 | 4.2 |
| ��ȫ���� | 3.2 | 8.9 | 6.7 |
��2����Ӧ���м���H2O2��������ʹFe2+����ΪFe3+��
��3�����ɳ�����Ӧ�����ӷ���ʽ��Fe3++3OH��=Fe��OH��3����
��4��������IJ����Ǽ���Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ����
��5���ⶨ�������崿�ȵ�ʵ�鲽�����£�
A�� ȷ��ȡ3.125g����������Ʒ���100mL��Һ��
B�� ȡ10.00mL��Һ�ڴ�����ƿ�У�������ˮϡ�ͣ��������KI���壬������Ӧ��2Cu2++4I��=2CuI��+I2
C�� ����������������У���μ���0.1000mol•L-1Na2S2O3��Һ��ǡ����ȫ��Ӧ��������12�� 00mL Na2S2O3��Һ��I2+2S2O32-=2I��+S4O62-������Ʒ�е����������������96%��
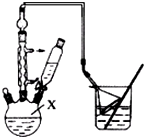
 ij��ѧʵ��С����0.2000 mol/L������KMnO4��Һ�ⶨ���ᾧ��Ĵ��ȣ����ᾧ�廯ѧʽΪH2C2O4•2H2O�����ʲ���KMnO4��Ӧ����ʵ�鲽�����£�
ij��ѧʵ��С����0.2000 mol/L������KMnO4��Һ�ⶨ���ᾧ��Ĵ��ȣ����ᾧ�廯ѧʽΪH2C2O4•2H2O�����ʲ���KMnO4��Ӧ����ʵ�鲽�����£���1����ȡ13.0 g���ᾧ�壬���250.00 mLˮ��Һ���˲������������õ��������ǣ���ƽ�������룩���ձ���ҩ�ס�����������ͷ�ιܺ�250 mL����ƿ
��2����ȡ������Һ25.00 mL������ƿ�У���0.2000 mol/L������KMnO4��Һ�ζ���
����ȡ25.00 mL������Һ��������25mL��ʽ�ζ��ܣ���25mL��Һ�ܣ���
�ڵζ�ʱ��KMnO4��ҺӦװ����ʽ �����ʽ��������ʽ�����ζ����У�
�۵ζ�ʱ��������Ӧ�����ӷ���ʽ��5H2C2O4+2MnO4-+6H+=2Mn2++10CO2��+8H2O��
�ܵζ��ﵽ�յ�ı�־�Ǽ������һ�θ��������Һ����ƿ����Һ����ɫ��Ϊ��ɫ���Ұ���Ӳ���ɫ��
��3������ȷ��������й����ݼ�¼���£�
| �ζ����� | ������Һ��� | ����KMnO4��Һ��� | |
| �ζ�ǰ����/m L | �ζ������/m L | ||
| ��һ�� | 25.00 | 0.20 | 20.58 |
| �ڶ��� | 25.00 | 4.00 | 24.40 |
| ������ | 25.00 | 2.38 | a |
�ڲ��ᾧ��Ĵ���Ϊ98.86%�����������λС������
��4��������ʵ���У����в���������������ȷ��һ������ɲⶨ���ƫ�ߵ���BD��
A����ȡ13.0 g���ᾧ��ʱ�������ᾧ�����������ƽ����
B����ƿˮϴ���ò�����Һ��ϴ
C����ȡKMnO4��Һ���ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ���
D��ʢKMnO4��Һ����ʽ�ζ��ܼ��첿�������ݣ��ζ���������ʧ��
aA��g��+bB��g��?2C��g��
| ��ʼ���ʵ���Ũ�ȣ�mol/L�� | 1.5 | 1 | 0 |
| 2sĩ���ʵ���Ũ�ȣ�mol/L�� | 0.9 | 0.8 | 0.4 |
��0��2s������B����ʾ�ķ�Ӧ����Ϊ0.1mol/��L•s����
�۴ӷ�Ӧ��ʼ��2sĩ��A��ת����Ϊ40%��
��������ʵ�ܹ�˵��������Ӧ�ڸ��������Ѿ��ﵽ��ѧƽ��״̬����BE��
A��vB����Ӧ��=vC�����ɣ� B���������������ѹǿ���ֲ���
C��������������ܶȲ��� D��vA��vB��vC=3��2��2
E������������C�����ʵ����������ֲ��䣮
 ���嶡�����ӣ�
���嶡�����ӣ�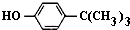 ����ҵ��;�㷺�����������������Է�ȩ��֬���ȶ��������ϵȣ�ʵ�����Ա��ӡ��嶡����[��CH3��3CCl]��Ϊԭ���Ʊ����嶡�����ӣ�ʵ�鲽�����£�
����ҵ��;�㷺�����������������Է�ȩ��֬���ȶ��������ϵȣ�ʵ�����Ա��ӡ��嶡����[��CH3��3CCl]��Ϊԭ���Ʊ����嶡�����ӣ�ʵ�鲽�����£� ��
��