��Ŀ����
17����Ϣʱ�������Ĵ������������Ի�������������в��ij�о���ѧϰС�齫һ����������·������õ���Cu��Fe������Au��Pt�Ƚ����Ļ����������������Ʊ��������壨CuSO4•5H2O����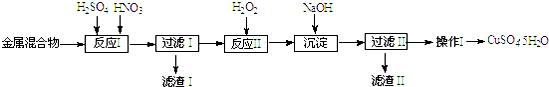
��֪������������������������ʽ����ʱ��Һ��pH���±���
| ������ | Fe3+ | Fe2+ | Cu2+ |
| ��ʼ���� | 1.5 | 6.4 | 4.2 |
| ��ȫ���� | 3.2 | 8.9 | 6.7 |
��2����Ӧ���м���H2O2��������ʹFe2+����ΪFe3+��
��3�����ɳ�����Ӧ�����ӷ���ʽ��Fe3++3OH��=Fe��OH��3����
��4��������IJ����Ǽ���Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ����
��5���ⶨ�������崿�ȵ�ʵ�鲽�����£�
A�� ȷ��ȡ3.125g����������Ʒ���100mL��Һ��
B�� ȡ10.00mL��Һ�ڴ�����ƿ�У�������ˮϡ�ͣ��������KI���壬������Ӧ��2Cu2++4I��=2CuI��+I2
C�� ����������������У���μ���0.1000mol•L-1Na2S2O3��Һ��ǡ����ȫ��Ӧ��������12�� 00mL Na2S2O3��Һ��I2+2S2O32-=2I��+S4O62-������Ʒ�е����������������96%��
���� ��Cu��Fe������Au��Pt�Ƚ����Ļ�����������������ܽ�ͭ��������Ͳ����ܽ⣬���˵õ�����ΪAu��Pt����Һ�м����������������������Ϊ�����ӣ���������������Һ������Һ��PH��3.2--4.2��Χ�ڣ����������ӣ����˵õ�������Ϊ������������ҺΪ����ͭ��Һ��ͨ������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����õ��������壬
��1�������Ļ�����м�����������ᣬCu��Fe�ᷢ����Ӧ��Ϊ��Һ��Au��Pt���ܽ⣻
��2����Ӧ���м���H2O2�������ǽ���Һ��Fe2+����ΪFe3+�����ڽ�����FeԪ�س�ȥ��
��3�����ɳ�����Ӧ��������������������������
��4���������Ǵ���Һ�еõ����ʾ��壬����IJ����Ǽ���Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ����
��5��2CuSO4•5H2O��2Cu2+��2I����I2��2S2O32-��n��S2O32-��=0.1mol/L��0.012L��10=0.012mol������õ�����ͭ�������ʵ���������õ���Ʒ�е������������������
��� �⣺��1����Cu��Fe������Au��Pt�Ƚ����Ļ�����м������������Cu��Fe�ᷢ����Ӧ��Ϊ��Һ��Au��Pt���ܽ⣬�������������Ҫ�ɷ���Au��Pt��
�ʴ�Ϊ��Au��Pt��
��2����Ӧ���м���H2O2�������ǽ���Һ��Fe2+����ΪFe3+��2Fe2++H2O2+2H+=2Fe3++2H2O��ͨ��������ҺPH���ڽ�����FeԪ�س�ȥ��
�ʴ�Ϊ��ʹFe2+����ΪFe3+��
��3�����ɳ�����Ӧ�����ӷ���ʽ�У�Fe3++3OH��=Fe��OH��3����
�ʴ�Ϊ��Fe3++3OH��=Fe��OH��3����
��4��������������ͭ��Һ�еõ�����ͭ���壬�����IJ����Ǽ���Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����ʴ�Ϊ������Ũ������ȴ�ᾧ��
��5��2Cu2++4I��=2CuI��+I2��I2+2S2O32-=2I��+S4O62-��
2CuSO4•5H2O��2Cu2+��2I����I2��2S2O32-��
n��S2O32-��=0.1mol/L��0.012L��10=0.012mol��
����n��CuSO4•5H2O��=0.012mol��
��������0.012mol��250g/mol=3.0g��
������Ʒ�е������������������$\frac{3.0g}{3.125g}$��100%=96%��
�ʴ�Ϊ��96%��
���� ���⿼��Ԫ�ؼ�����������ʡ����ʵij�ȥ�����ӷ���ʽ����д����ϵʽ���ڻ�ѧ�����е�Ӧ�ü����ʴ��ȵļ����֪ʶ�����ջ����ǽ���ؼ�����Ŀ�Ѷ��еȣ�

 �ظ���ʦ�㲦ϵ�д�
�ظ���ʦ�㲦ϵ�д�| A�� | 1 mol H2SO4��������98 g/mol | |
| B�� | CO2��Ħ����������CO2����Է������� | |
| C�� | Ħ���������������ʵ����ʵ��������ʵ�����֮�����ϵ | |
| D�� | 1 mol�κ����ʵ��������ڸ����ʵ���Է������� |
�ٵζ��յ�ʱ������Ϊ��ƿ�е���Һ�ɺ�ɫ����ɫ���ڰ�����ڲ��ָ���
�ڵζ�ʱ�ߵα�ҡ����ƿ���۾�Ӧ�۲���ƿ����Һ��ɫ�ı仯��
�����в�������ʹ�ⶨ���ƫ�͵���C��
A����ʽ�ζ�����װ��Һǰδ�ñ���Һ��ϴ
B����ʼʱ��ʽ�ζ��ܼ��첿���������ݣ��ζ���������ʧ
C��װ����Һ�ĵζ��ܣ���Һǰƽ�ӣ�������
D��ʢNaOH��Һ����ƿ�ζ�ǰ��NaOH��Һ��ϴ2��3��
����֪����������ݣ�
| �ⶨ���� | ����Һ���/mL | ���������/mL | |
| �ζ�ǰ����/mL | �ζ������/mL | ||
| ��һ�� | 25.00 | 0.40 | 20.38 |
| �ڶ��� | 25.00 | 4.00 | 24.02 |
 ��ʹ������к͵ζ����ⶨ���۰״���������g•100mL-1����
��ʹ������к͵ζ����ⶨ���۰״���������g•100mL-1������ʵ�鲽��
��1������ʽ�ζ��ܣ����������ƣ���ȡ10.00mLʳ�ð״ף����ձ�����ˮϡ�ͺ�ת�Ƶ�100mL����ƿ�����������ƣ��ж��ݣ�ҡ�ȼ��ô���״���Һ��
��2������ʽ�ζ���ȡ����״���Һ20.00mL����ƿ�У������еμ�2�η�̪��ָʾ����
��3����ȡʢװ0.100 0mol•L-1 NaOH ��Һ�ļ�ʽ�ζ��ܵij�ʼ���������Һ��λ����ͼ��ʾ�����ʱ�Ķ���Ϊ0.60mL��
��4���ζ�������Һ����ɫǡ�ñ�Ϊdz��ɫ�����ڰ�����ڲ���ɫʱ��ֹͣ�ζ�������¼NaOH��Һ���ն������ظ��ζ�3�Σ�
��ʵ���¼
| �ζ�����ʵ�����ݣ�mL�� | 1 | 2 | 3 | 4 |
| V����Ʒ�� | 20.00 | 20.00 | 20.00 | 20.00 |
| V��NaOH�������ģ� | 15.95 | 15.00 | 15.05 | 14.95 |
��1����ͬѧ�ڴ�������ʱ����ã�
ƽ�����ĵ�NaOH��Һ�����V=$\frac{15.95+15.00+15.05+14.95}{4}$mL=15.24mL��ָ�����ļ���IJ�����֮����һ��������Դ����쳣ֵ��Ӧ��ȥ������ȷ���ݴ������ɵ�c�����۰״ף�=0.45mol•L-1�����۰״�������=0.075g•100mL-1��
��2���ڱ�ʵ��ĵζ������У����в�����ʹʵ����ƫ�����ab��д��ţ���
a����ʽ�ζ����ڵζ�ʱδ�ñ�NaOH��Һ��ϴ
b����ʽ�ζ��ܵļ����ڵζ�ǰ�����ݣ��ζ���������ʧ
c����ƿ�м������״���Һ���ټ�����ˮ
d����ƿ�ڵζ�ʱ����ҡ����������Һ�彦����
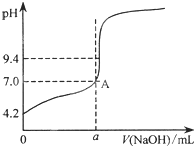 �����ڱ���������ر���Һ���ⶨNaOH��Һ��Ũ�ȣ�������������Һ���ζ��ڱ���������Һʱ�����в�����
�����ڱ���������ر���Һ���ⶨNaOH��Һ��Ũ�ȣ�������������Һ���ζ��ڱ���������Һʱ�����в�����������Һ�м���1��2��ָʾ�� ��ȡ20mL����Һ������ƿ��
��������������Һ�ζ����յ� ���ظ����ϲ���
������ƽ��ȷ��ȡ5.105g�ڱ���������أ���Է�������Ϊ204.2���������250mL����Һ�����pHԼΪ4.2����
����ʵ�����ݼ����������Ƶ����ʵ���Ũ�ȣ�
��1�����ϸ����У���ȷ�ģ�����ţ�����˳���Ǣݢڢ٢ۢܢޣ���������ʹ�õ���������ƿ�⣬����Ҫʹ�õ���������ʽ�ζ��ܣ�ѡ��ָʾ���ǣ���̪
��2���ζ�������¼NaOH���ն������ظ��ζ����Σ����ݼ�¼���±���
| �ζ����� ʵ������ | 1 | 2 | 3 | 4 |
| V����Ʒ��/mL | 20.00 | 20.00 | 20.00 | 20.00 |
| V��NaOH��/mL���������� | 0.10 | 0.30 | 0.00 | 0.20 |
| V��NaOH��/mL���ն����� | 20.08 | 20.30 | 20.80 | 20.22 |
| V��NaOH��/mL�����ģ� | 19.98 | 20.00 | 20.80 | 20.02 |
V��NaOH��=$\frac{19.98+20.00+20.80+20.02}{4}$20.20mL�����ļ�������������Dz���������3�����ݺ������������ϴ�Ӧ����
ͨ��������õ�4�εζ���������ҺpH������� ��������Һ����ı仯������ͼ��ʾ����a��20.02�����������������=����
��3����������ڹ۲�ζ��ܵ���ʼ����ʱ��Ҫʹ�ζ��ܵļ��첿�ֳ�����Һ������ζ����ڲ������ݣ��������ݵIJ������ٷ�Һ��
��4���ζ�ǰ��������ˮϴ����ʽ�ζ��ܣ�Ȼ��Ӵ��ⶨ������������Һ�ζ����˲�����ʵ����ƫС���ƫ����ƫС������Ӱ�족��
��1���õ绡���ϳɵĴ�������̼�ܳ����д�����̼�����������ʣ������ֿ������������������ᴿ������ɸ÷�Ӧ�Ļ�ѧ����ʽ��
5C+4KMnO4+6H2SO4=5CO2��+4MnSO4+2K2SO4+6H2O
��2����2L�ܱ������м���NO�ͻ���̿�������ʣ�����������E��F�����¶ȷֱ���T1��T2ʱ����ø�����ƽ��ʱ���ʵ��������
| n/mol T/�� | ����̿ | NO | E | F |
| ��ʼ | 2.030 | 0.100 | 0 | 0 |
| T1 | 2.000 | 0.040 | 0.030 | 0.030 |
| T2 | 2.005 | 0.050 | 0.025 | 0.025 |
�ټ��㣺K1=$\frac{9}{16}$��
�ڸ���������Ϣ�жϣ��¶�T1��T2�Ĺ�ϵ�ǣ�����ţ�C��
A�� T1��T2 B�� T1��T2 C�����Ƚ�
��3����ҵ������CO��ˮ������һ�������·�����Ӧ��ȡ������CO��g��+H2O��g��?CO2��g��+H2��g������H=-41kJ/mol
��֪��2H2O ��g���T2H2��g��+O2��g������H=+484kJ/mol��
��д��CO��ȫȼ������CO2���Ȼ�ѧ����ʽ��2CO��g��+O2��g���T2CO2��g����H=-566 kJ/mol��
��ij�¶��£���һ�ݻ��ɱ�������У�COת������CO2�ķ�Ӧ�ﵽƽ��ʱ��CO��O2��CO2�����ʵ����ֱ�Ϊ4mol��2mol����4mol�������¶Ⱥ�ѹǿ���䣬��ƽ�����������ߵ����ʵ��������µ�������ʹƽ�����Ƶ��ǣ�������ţ�A��
A�� ������1mol B�� ���ӱ� C�� ������1mol D�� �����룮
 Na2S2O3����Ҫ�Ļ���ԭ�ϣ�������ˮ�������Ի���Ի������ȶ���
Na2S2O3����Ҫ�Ļ���ԭ�ϣ�������ˮ�������Ի���Ի������ȶ���
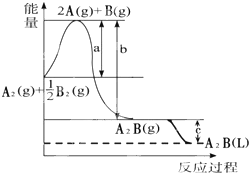 ��֪��A2��g��+$\frac{1}{2}$B2��g���TA2B��g������Ӧ�����������仯��ͼ���ʣ�
��֪��A2��g��+$\frac{1}{2}$B2��g���TA2B��g������Ӧ�����������仯��ͼ���ʣ�