��Ŀ����
6����֪ˮ��25���95��ʱ�������ƽ��������ͼ��ʾ��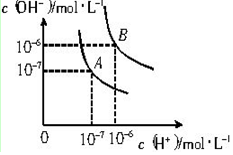
��1����25��ʱˮ�ĵ���ƽ������ӦΪA���A����B��������˵������ˮ�ĵ��������ȹ��̣��¶ȵ�ʱ��ˮ�ĵ���̶�С��c��H+����c��OH-��С��
��2��95��ʱ����10���pH1=a��ijǿ����Һ��1���pH2=b��ijǿ����Һ��Ϻ���Һ�����ԣ�����ǰ����ǿ���pH1��ǿ���pH2֮��Ӧ����Ĺ�ϵ��a+b=13�� pH1+pH2=13��
��3��25��ʱ����pH=11��NaOH��Һ��pH=4��H2SO4��Һ��ϣ������û����Һ��pH=10����NaOH��Һ��H2SO4��Һ�������Ϊ2��9��
��4������B��Ӧ�¶��£�pH=2��ijHA��Һ��pH=10��NaOH��Һ�������Ϻ����Һ��pH=5���������ԭ������B��Ӧ95�棬��ʱˮ�����ӻ�Ϊ10-12��HAΪ���ᣬHA��NaOH�кͺ����Һ�л�ʣ��϶��HA���ӣ��ɼ��������H+��ʹ��ҺpH=5��
���� ��1��������������Ũ�ȣ�����������������Ũ�ȣ�ˮ�����ӻ�����Kw=c��H+����c��OH-�������A���ߵ�Kw��Ȼ����ˮ�ĵ�����������ж�25��ʱˮ�ĵ���ƽ�����ߣ�
��2���������Һ��pHΪa������Һ��pHΪb�����ݸ��¶��Լ������ϵ��ʽ���㣻
��3��pH=4��H2SO4��Һ�������ӵ����ʵ���Ũ��Ϊ0.0001mol/L��pH=11��NaOH��Һ�����������ӵ����ʵ���Ũ��Ϊ��10-3mol/L���������Һ��pH=10ʱ����Һ�ʼ��ԣ����������ƹ������ݴ���ʽ���㣻
��4����������B��Ӧ�¶���pH=5��˵����Һ��ʾ���ԣ���Ӧ�������ӹ���������
��� �⣺��1������A������Kw=c��H+����c��OH-��=10-7��10-7=10-14������B������c��H+��=c��OH-��=10-6 mol/L��Kw=c��H+��•c��OH-��=10-12��ˮ�ĵ���ʱ���ȹ��̣����ȴٽ����룬����A���ߴ���25��ʱˮ�ĵ���ƽ�����ߣ��ʴ�Ϊ��A��ˮ�ĵ��������ȹ��̣��¶ȵ�ʱ��ˮ�ĵ���̶�С��c��H+����c��OH-��С��
��2����ǿ����Һ��pHΪa�����Ϊ10V����Һ��������Ũ��Ϊ��10-amol/L������Һ��pHΪb�����ΪV����Һ�����������ӵ�Ũ��Ϊ��10-��12-b��mol/L��
��Ϻ���Һ�����ԣ���������Һ�������ӵ����ʵ����������������ӵ����ʵ�������10-amol/L��10VL=10-��12-b��mol/L��VL��
��ã�1-a=b-12��a+b=13����pH1+pH2=13��
�ʴ�Ϊ��a+b=13�� pH1+pH2=13��
��3��pH=4��H2SO4��Һ�������ӵ����ʵ���Ũ��Ϊ0.0001mol/L��pH=11��NaOH��Һ�����������ӵ����ʵ���Ũ��Ϊ��10-3mol/L�������û����Һ��pH=10����Ӧ�����Һ��c��OH-��=0.0001��mol/L����
��c��OH-��=$\frac{0.001V��-0.0001V��}{V��+V��}$=0.0001mol/L��
��ã�V����V��=2��9���ʴ�Ϊ��2��9��
��4��������B��Ӧ�¶��£���pH���ᣩ+pH���=12���ɵ��������Һ��c��H+��=c��OH-��������ǿ������Һ�������Ϻ���Һ�����ԣ��ֻ����Һ��pH=5�����������Ϻ���Һ�����ԣ�˵��H+��OH-��ȫ��Ӧ�������µ�H+�������������������HA�����ᣬ
�ʴ�Ϊ������B��Ӧ95�棬��ʱˮ�����ӻ�Ϊ10-12��HAΪ���ᣬHA�к�NaOH�����Һ�л�ʣ��϶��HA���ӣ��ɼ��������H+��ʹ��ҺpH=5��
���� ���⿼����ˮ�ĵ��롢ˮ�����ӻ�����ҺpH�ļ��㣬��Ŀ�Ѷ��еȣ�����ؼ����ڸ�����¶ȶ�ˮ����ƽ�⡢ˮ�����ӻ�����ҺpH��Ӱ�죮

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | һ���¶���Һ����c��NH4+��•c��NH2-����һ������ | |
| B�� | Һ���к���NH3��NH4+��NH2-������ | |
| C�� | ֻҪ�������������ʣ�Һ����c��NH4+��=c��NH2-�� | |
| D�� | Һ���ﵽ����ƽ��ʱc��NH3��=c��NH4+��=c��NH2-�� |
| A�� | BaCl2��ϡ���� | B�� | AgNO3��ϡ���� | C�� | ϡ���ᡢBaCl2 | D�� | AgNO3��ϡ���� |
| ʱ��/s | 0 | 20 | 40 | 60 | 80 |
| c��N2O4��/mol•L-1 | 0.100 | c1 | 0.050 | c3 | c4 |
| c��NO2��/mol•L-1 | 0.000 | 0.060 | c2 | 0.120 | 0.120 |

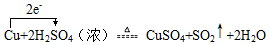 ��ʵ���ã���Ӧ�������˱�״���µ�SO2����44.8L����������������ʵ���Ϊ2mol��
��ʵ���ã���Ӧ�������˱�״���µ�SO2����44.8L����������������ʵ���Ϊ2mol��