��Ŀ����
2�����仯�������ִ���ҵ�����������������Ҫ��Ӧ�ü�ֵ��
��1����̬��ԭ�ӵĵ����Ų�ʽ��1s22s22p1�����������B2H6�������飩���ṹ��ͼ1��ʾ������Bԭ�ӵ��ӻ���ʽΪsp3�ӻ���
��2�����Ȼ���������������¶������壬����ǿ�ҵĽ��ܹµ��ӶԵ������Ʋ����ǹ�̬ʱ�ľ�������Ϊ���Ӿ��壻���������백���������������ɰ�ɫ���壬д���ð�ɫ����ṹʽ������ע�����е���
�
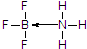 ��
����3�����ṹ�о�֤�������ᾧ����B��OH��3��Ԫ�ṹ��ͼ����ʾ������Ԫ�е���ԭ��ͨ��O-H��O�������ɲ�״�ṹ����Ƭ��ṹ������������������ͼ����ʾ�������֮�������ķ��Ӽ������Ϲ����������ᾧ�壮
��H3BO3��һԪ���ᣬд������ˮ��Ӧ�Ļ�ѧ����ʽH3BO3+H2O?[B��OH��4]-+H+��
�ڸ��ݽṹ�ж�����˵����ȷ����ac��
a�����ᾧ���л���У������� b��H3BO3���ӵ��ȶ���������й�
c����1mol H3BO3�ľ�������3mol��� d��H3BO3��������ԭ�������Ϊ8e-�ȶ��ṹ
��4�����á�±�����ɺϳɺ�B��N����Ԫ�صĹ����մɣ�ͼ2Ϊ�侧���ṹʾ��ͼ��
�ٸù����մɵĻ�ѧʽΪBN���ڵ�һ�����ܽ���B��N֮��ĵڶ�����Ԫ����3�֣�
���� ��1������Ԫ�����ƣ��ж�Ԫ��ԭ�ӵĺ�����������ٸ��ݺ�������Ų�������д����Ԫ��Ϊ5��Ԫ�أ����ݼ۲���ӶԻ�������ȷ�����ӻ����ͣ�
��2�����ݾ�����۷е���жϾ������ͣ���Ԫ�ؾ���ȱ�����ԣ��仯���������йµ��ӶԵķ��ӻ������γ�����BF3����NH3��Ӧ����BF3•NH3��Nԭ�Ӻ��йµ��Ӷԣ�
��3��������ΪһԪ���ᣬ��ˮ��Һ���ˮ����γ���λ�������ֵ�����������ӣ�
��a���������ᾧ��ΪƬ��״�ṹ������
b�����ӵ��ȶ����뻯ѧ���йأ�
c�����þ�̯�����㺬1molH3BO3�ľ����е��������1mol H3BO3�ľ�������3mol�����
d���ɽṹ��֪����ԭ�������ֻ��3�����ӣ�����ԭ���γ�3�Թ��õ��Ӷԣ�
��4�������þ�̯������ԭ�Ӹ���������Ԫ��ԭ�Ӹ�������ȷ����ѧʽ��
��ͬһ����Ԫ���У�Ԫ�صĵ�һ����������ԭ��������������������ƣ�����IIA�塢��VA��Ԫ�ص�һ�����ܴ���������Ԫ�أ�
��� �⣺��1��BԪ��Ϊ5��Ԫ�أ�ԭ�Ӻ�����5�����ӣ��������Ų�����һ��2�����ڶ���3�������Ժ�������Ų�ʽΪ��1s22s22p1�������������ÿ����ԭ�Ӻ���4�����ۼ�������Bԭ�Ӳ���sp3�ӻ���
�ʴ�Ϊ��1s22s22p1��sp3�ӻ���
��3�����Ȼ���������������¶������壬�������ǹ�̬ʱ�ľ�������Ϊ���Ӿ��壬��Ԫ�ؾ���ȱ�����ԣ��仯���������йµ��ӶԵķ��ӻ������γ�����BF3����NH3��Ӧ����BF3•NH3��B��N֮���γ���λ����Nԭ�Ӻ��йµ��Ӷԣ����Ե�ԭ���ṩ�µ��Ӷԣ�BF3•NH3�ṹʽΪ��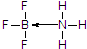 ��
��
�ʴ�Ϊ�����Ӿ��壻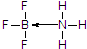 ��
��
��3��������ΪһԪ���ᣬ��ˮ��Һ���ˮ������������������γ���λ��������뷽��ʽΪ��H3BO3+H2O?[B��OH��4]-+H+��
�ʴ�Ϊ��H3BO3+H2O?[B��OH��4]-+H+��
��a�����ᣨH3BO3����һ��Ƭ��״�ṹ��ɫ���壬Ƭ��״�ṹ�����л���У�����������a��ȷ��
b�����ӵ��ȶ���������ڵ�B-O��H-O���ۼ��йأ��۷е�������йأ���b����
c��1����������γ���6���������ÿ�������2��������ӹ��õģ�����ƽ����3�����������1molH3BO3�ľ�������3mol�������c��ȷ��
d����ԭ�������ֻ��3�����ӣ�����ԭ���γ�3�Թ��õ��Ӷԣ����Bԭ�Ӳ���8e-�ȶ��ṹ����d����
�ʴ�Ϊ��ac��
��4���ٸ���ԭ�Ӱ뾶��С֪��Bԭ�Ӱ뾶����Nԭ�ӣ�����ɫ���ʾBԭ�ӣ����þ�̯����Bԭ�Ӹ���=1+8��$\frac{1}{8}$=2��Nԭ�Ӹ���=1+4��$\frac{1}{4}$=2��Bԭ�Ӻ�Nԭ�Ӹ���֮��Ϊ2��2=1��1�������仯ѧʽΪBN��
�ʴ�Ϊ��BN��
��ͬһ����Ԫ���У�Ԫ�صĵ�һ����������ԭ��������������������ƣ�����IIA�塢��VA��Ԫ�ص�һ�����ܴ���������Ԫ�أ����Ե�һ�����ܽ���B��N֮��ĵڶ�����Ԫ����Be��C��O��������3�֣�
�ʴ�Ϊ��3��
���� ���⿼�����й����֪ʶ�����ؿ���ԭ�Ӻ�������Ų����ӻ����۵�Ӧ�á����ᾧ�徧�����͵��жϡ�Ӱ������ȶ��Ե����ص�֪ʶ�㣬��Ŀ�Ѷ��еȣ�ע����ӵ��ȶ����뻯ѧ���йأ����ʵ��۷е�������йأ�ע�����ᣨH3BO3����һ��Ƭ��״�ṹ��ɫ���壮

 ���źþ���Ԫ����ĩ��ϵ�д�
���źþ���Ԫ����ĩ��ϵ�д�| A�� | ��״���£�1 mol CCl4�����ԼΪ22.4L | |
| B�� | NaOH��Ħ��������40g | |
| C�� | ���³�ѹ�£�11.2L����������ͭ�۳�ַ�Ӧ��ת�Ƶĵ�����Ϊ2NA | |
| D�� | 46g NO2 ��46g N2O4 ���е�ԭ������Ϊ3NA |
| A�� | C���ĵ�������A���ĵ�������� | |
| B�� | ��λʱ��������a mol B��ͬʱ����a mol C | |
| C�� | �����ڵ�ѹǿ���ٱ仯 | |
| D�� | ���������ܶȲ��ٱ仯 |
| A�� | KHCO3��MgCO3 | B�� | K2CO3��Na2CO3 | C�� | MgCO3��Na2CO3 | D�� | KHCO3��NaHCO3 |

��֪���ٽ���Һ���е���������Ҫ��H+��Co2+��Fe2+��Mn2+��Al3+�ȣ�
�ڲ���������������������ʽ����ʱ��Һ��pH����������������Ũ��Ϊ��0.01mol/L��
| ������ | Fe��OH��3 | Fe��OH��2 | Co��OH��2 | Al��OH��3 | Mn��OH��2 |
| ��ʼ���� | 2.7 | 7.6 | 7.6 | 4.0 | 7.7 |
| ��ȫ���� | 3.7 | 9.6 | 9.2 | 5.2 | 9.8 |
��1��ˮ�ܿ����Ԥ����ʱ����Na2SO3����Ҫ�����ǽ�Fe3+��Co3+��ԭ��
��2��д��NaClO3�ڽ���Һ�з�����Ҫ��Ӧ�����ӷ���ʽClO3-+6Fe2++6H+=Cl-+6Fe3++3H2O������������Һ���мӹ���NaClO3ʱ�����ܻ������ж����壬д�����ɸ��ж���������ӷ���ʽClO3-+5Cl-+6H+=3Cl2��+3H2O��
��3����Na2CO3��pH��a��a��ȡֵ��Χ��5.2��a��7.6���Ƶõ�CoCl2•6H2O�ں��ʱ���ѹ��ɵ�ԭ���ǽ��ͺ���¶ȣ���ֹ��Ʒ�ֽ⣮
��4����ȡ���Խ������ӵ���ȡ����pH�Ĺ�ϵ��ͼ������Һ���м�����ȡ����Ŀ���dz�ȥ��Һ�е�Mn2+����ʹ�õ����pH��Χ��B��
A��2.0��2.5
B��3.0��3.5
C��4.0��4.5
D��5.0��5.5
��5��CoCl2•6H2O�ܽ�����¶����������������ôֲ�Ʒͨ���ؽᾧ�����ᴿ��

 ����������ֳ�ΪĦ���Σ���һ����Ҫ�Ļ���ԭ�ϣ�����ɿɱ�ʾΪx��NH4��2S04•yFeS04•zH20��ij��ȤС��̽������е�x��y��z����ֵ��
����������ֳ�ΪĦ���Σ���һ����Ҫ�Ļ���ԭ�ϣ�����ɿɱ�ʾΪx��NH4��2S04•yFeS04•zH20��ij��ȤС��̽������е�x��y��z����ֵ��