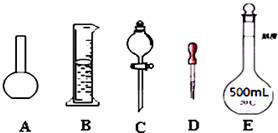
��Ŀ����
4��ij��ѧ����С���ú���Ϊԭ����ȡ�������ĵ�ˮ���������Ȼ�̼�ӵ�ˮ����ȡ�Ⲣ�÷�Һ©������������Һ����ʵ������ɷֽ�Ϊ���¼�����A����ʢ����Һ�ķ�Һ©����������̨����Ȧ�У�
B����50mL��ˮ��15mL���Ȼ�̼�����Һ©���У����Ǻò�������
C�������Һ©�������ϵIJ������Ƿ�©Һ��
D����ת©������������ʱ�����������������رջ������ѷ�Һ©��������
E���������������ձ�������Һ��
F���ӷ�Һ©�����Ͽڵ����ϲ�ˮ��Һ��
G����©���ϿڵIJ�������ʹ���ϵİ��ۻ�С��©�����ϵ�С�ף�
H�����ã��ֲ㣮
�ʹ�ʵ�飬���������գ�
��1����ȷ���������˳���ǣ����������������ı����ĸ��д��
C��B��D��A��H��G��E��F��
��2������E����IJ�����Ӧע��ʹ©���¶˹ܿڽ����ձ��ڱڣ���ʱ�رջ�������Ҫ���ϲ�Һ��������
��3������G���������Ŀ����ʹ©�����������ͨ���Ա�֤����E����ʱ��©���ڵ�Һ����˳��������
��4����֪���ڱ��е��ܽ�ȱ���ˮ�д�ö࣬�ܲ����ñ�����ȡ��ˮ�еĵ��ܣ���ܡ����ܡ������������DZ���ˮ�����ܣ����ҵ��ڱ����ܽ�ȱ���ˮ�д�ö࣬���뱽����Ӧ��
���� ��1������Ϊ��©��װҺ�������á���Һ��
��2�����������������²�Һ�壻��G�������ʹҺ��˳�����£�
��3�����ݲ���Ŀ�ķ�����
��4������ˮ�����Ȼ�̼�е��ܽ��Բ�ͬ��
��� �⣺��1������Ϊ��©��װҺ�������á���Һ������ΪC��B��D��A��H��G��E��F��
�ʴ�Ϊ��C��B��D��H��E��F��
��2�����������������²�Һ�壬Ӧʹ©���¶˹ܿڽ����ձ��ڱڣ���ʱ�رջ�������Ҫ���ϲ�Һ����������G������Ŀ��Ϊ����ʱ©����Һ���ܹ�������
�ʴ�Ϊ��ʹ©���¶˹ܿڽ����ձ��ڱڣ���ʱ�رջ�������Ҫ���ϲ�Һ��������
��3����G�����������Ŀ����Ϊ��ʹ©�����������ͨ���Ա�֤����Һ��˳��������
�ʴ�Ϊ��ʹ©�����������ͨ���Ա�֤���У�E������ʱ©����Һ���ܹ�������
��4������ˮ�����ܣ����ҵ��ڱ����ܽ�ȱ���ˮ�д�ö࣬���뱽����Ӧ��
�ʴ�Ϊ���ܣ�����ˮ�����ܣ����ҵ��ڱ����ܽ�ȱ���ˮ�д�ö࣬���뱽����Ӧ��
���� ���⿼���˷�Һ���й�ʵ��������⣬ע��ʵ������IJ��裬�����ѶȲ���

��ϰ��ϵ�д�
 ��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
�����Ŀ
2���������ӷ���ʽ��ȷ���ǣ�������
| A�� | ����������Һ�м��������ˮ��Al3++3OH-=Al��OH��3�� | |
| B�� | ��Ba��OH��2��Һ�еμ�NaHSO4��Һ�������Һǡ��Ϊ���ԣ�Ba2++OH-+H++SO42-=BaSO4��+H2O | |
| C�� | FeSO4��Һ��ϡ���ᡢ˫��ˮ��ϣ�2Fe2++H2O2+2H+=2Fe3++2H2O | |
| D�� | ��NaHCO3��Һ�м�������Ba��OH��2����Һ��Ba2++2HCO3-+2OH-=2H2O+BaCO3��+CO32- |
3���������ʵ����ѡ���װ�û��������ܴﵽʵ��Ŀ���ǣ�������
| A�� |  ��ȡ����ˮ | B�� |  ��ȡNH3 | C�� |  �ռ�NO2 | D�� |  ����ˮ�;ƾ� |
7��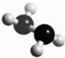 �£�N2H4���ǻ��ȼ�ϣ�����ӵ����ģ����ͼ��ʾ��������H2O2������Ӧ��N2H4+2H2O2�TN2+4H2O����NAΪ����٤��������ֵ������˵����ȷ���ǣ�������
�£�N2H4���ǻ��ȼ�ϣ�����ӵ����ģ����ͼ��ʾ��������H2O2������Ӧ��N2H4+2H2O2�TN2+4H2O����NAΪ����٤��������ֵ������˵����ȷ���ǣ�������
 �£�N2H4���ǻ��ȼ�ϣ�����ӵ����ģ����ͼ��ʾ��������H2O2������Ӧ��N2H4+2H2O2�TN2+4H2O����NAΪ����٤��������ֵ������˵����ȷ���ǣ�������
�£�N2H4���ǻ��ȼ�ϣ�����ӵ����ģ����ͼ��ʾ��������H2O2������Ӧ��N2H4+2H2O2�TN2+4H2O����NAΪ����٤��������ֵ������˵����ȷ���ǣ�������| A�� | 32g N2H4�к��й��ۼ�������Ϊ6NA | |
| B�� | ��״���£�22.4LH2O2������ԭ������Ϊ4NA | |
| C�� | 28g N2�к���������Ϊ7NA | |
| D�� | ��17g H2O2�����Ǽ��Լ���Ŀ��ͬ��N2H4�ķ�������Ϊ0.5NA |
9����һ�̶��ݻ����ܱ������У����淴ӦN2+3H2?2NH3�ﵽƽ��״̬�ı�־�ǣ�������
| A�� | v��N2����=v��NH3���� | |
| B�� | ��λʱ����3molH-H�����ѣ�ͬʱ6molN-H������ | |
| C�� | n��N2����n��H2������NH3��=1��3��2 | |
| D�� | ���������ܶȲ��ٸı� |
13������˵����ȷ���ǣ�������
| A�� | �ڹ��ۻ������в����ܺ������Ӽ� | |
| B�� | ��������֮��ͨ�����������γɵĻ�ѧ�����й��ۼ� | |
| C�� | ���й��ۼ��Ļ�����һ���ǹ��ۻ����� | |
| D�� | �������Ӽ��Ļ����ﲻһ�������ӻ����� |