��Ŀ����
6���к˵������1-18֮�������Ԫ��A��B��C��D��E�����ǵĺ˵����������������B��C��D���Ӳ�����ͬ��A��E������������ͬ����A��ĸ�Ԫ�ص�ԭ�ӵĵ��Ӳ��ڲ����������ӣ�����B���������4�����ӣ�C�������������Ǵ�����������3����D��E�γ����ӻ���������ӻ����������ǻ��ϼ۵ľ���ֵ��ȣ���ش�ע���漰A��B��C��D�Ļ�ѧ������Ԫ�ط��ű�ʾ������1��A�������⣬Dԭ�ӵĽṹʾ��ͼ
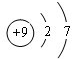 ��C2-�ĵ���ʽ
��C2-�ĵ���ʽ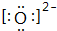 ��
����2��д��D��E�γɵ����ӻ�����ĵ��뷽��ʽNaF=Na++F-��
��3��B��C�м���N��дԪ�ط��ţ�����д���䵥�ʵĽṹʽN��N��
��4��E����������A2C��Ӧ�����������ƣ�д���ƣ�����д������ĵ���ʽ
 ��
����5��A��B�γɵ�ij��̬����������Ȼ������Ҫ�ɷ֣���֪1g�û�������ȼ������BO2�����Һ̬A2Oʱ�ų�55.6kJ��������д�ɸ÷�Ӧ���Ȼ�ѧ����ʽCH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-889.6kJ/mol��
���� �к˵������1-18֮�������Ԫ��A��B��C��D��E�����ǵĺ˵����������������A��E������������ͬ����A��ĸ�Ԫ�ص�ԭ�ӵĵ��Ӳ��ڲ����������ӣ����У�C�������������Ǵ�����������3����ԭ��ֻ����2�����Ӳ㣬����������Ϊ6����CΪOԪ�أ�B��C��D���Ӳ�����ͬ��B���������4�����ӣ���BΪ̼Ԫ�أ�D��ԭ��������������D��E�γ����ӻ���������ӻ����������ǻ��ϼ۵ľ���ֵ��ȣ���DΪFԪ�ء�EΪNa��A��E������������ͬ����A��ĸ�Ԫ�ص�ԭ�ӵĵ��Ӳ��ڲ����������ӣ���AΪHԪ�أ��ݴ˽��
��� �⣺�к˵������1-18֮�������Ԫ��A��B��C��D��E�����ǵĺ˵����������������A��E������������ͬ����A��ĸ�Ԫ�ص�ԭ�ӵĵ��Ӳ��ڲ����������ӣ����У�C�������������Ǵ�����������3����ԭ��ֻ����2�����Ӳ㣬����������Ϊ6����CΪOԪ�أ�B��C��D���Ӳ�����ͬ��B���������4�����ӣ���BΪ̼Ԫ�أ�D��ԭ��������������D��E�γ����ӻ���������ӻ����������ǻ��ϼ۵ľ���ֵ��ȣ���DΪFԪ�ء�EΪNa��A��E������������ͬ����A��ĸ�Ԫ�ص�ԭ�ӵĵ��Ӳ��ڲ����������ӣ���AΪHԪ�أ�
��1��A������Ϊ�⣬DΪFԪ�أ�ԭ�ӵĽṹʾ��ͼΪ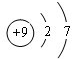 ��O2-�ĵ���ʽΪ
��O2-�ĵ���ʽΪ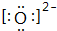 ��
��
�ʴ�Ϊ���⣻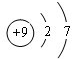 ��
��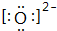 ��
��
��2��D��E�γɵ����ӻ�����ΪNaF�����뷽��ʽΪNaF=Na++F-��
�ʴ�Ϊ��NaF=Na++F-��
��3��B��C�м���NԪ�أ��䵥�ʵĽṹʽΪN��N��
�ʴ�Ϊ��N��N��N��
��4��E����������H2O��Ӧ�����������ƣ�����ʽΪ ��
��
�ʴ�Ϊ���������ƣ� ��
��
��5��A��B�γɵ�ij��̬�������������Ȼ������Ҫ�ɷ֣���֪1g������ȼ������CO2�����Һ̬H2Oʱ�ų�55.6kJ����������1mol������ȫȼ�շų�������Ϊ55.6kJ��$\frac{1mol��16g/mol}{1g}$=889.6kJ���÷�Ӧ���Ȼ�ѧ����ʽΪ��CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-889.6kJ/mol��
�ʴ�Ϊ��CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-889.6kJ/mol��
���� ���⿼��ṹ����λ�ù�ϵӦ�ã����ضԻ�ѧ����Ŀ��飬�ƶ�Ԫ���ǽ���ؼ���ע��Ի���֪ʶ���������գ�

| A�� | H2SO4��Һ | B�� | BaCl2��Һ | C�� | NaOH��Һ | D�� | AgNO3��Һ |
| A�� | ��ȥFe2O3��Al2O3������е�Fe2O3��NaOH��Һ | |
| B�� | ��ȥNa2O2�е�Na2O������ | |
| C�� | ��ȥNa2CO3�����е�NaHCO3����NaOH���� | |
| D�� | ��ȥFe��C�Ͻ��е�C����O2������ |
��pH=11�İ�ˮbL
�����ʵ���Ũ��Ϊ1��10-1mol•L-1�İ�ˮcL
��c��OH��=1��10-3mol•L-1��Ba��OH��2��ҺdL��
���ж�a��b��c��d��С��ϵ��ȷ��Ϊ��������
| A�� | c��a=d��b | B�� | b��a=d��c | C�� | c��a��b��d | D�� | a=b��c��d |
| A�� | NaOH��Һ | B�� | AgNO3��Һ | C�� | BaCl2��Һ | D�� | Ba��OH��2��Һ |
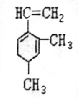 ��֪ij�л���Ľṹ��ʽ��ͼ�����Ǿ��л�״�ṹ�IJ���������
��֪ij�л���Ľṹ��ʽ��ͼ�����Ǿ��л�״�ṹ�IJ���������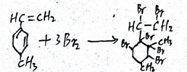 ��
��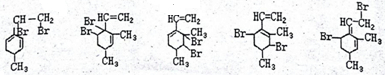 ��
��