��Ŀ����
17��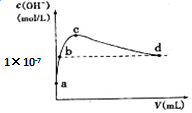 �����£���1 L pH=10��NaOH��Һ�г���ͨ��CO2��ͨ��CO2�������V������Һ��ˮ�������c��OH-���Ĺ�ϵ��ͼ��ʾ��������������ȷ���ǣ�������
�����£���1 L pH=10��NaOH��Һ�г���ͨ��CO2��ͨ��CO2�������V������Һ��ˮ�������c��OH-���Ĺ�ϵ��ͼ��ʾ��������������ȷ���ǣ�������| A�� | d����Һ�У�c��Na+���T2c��CO32-��+c��HCO3-�� | |
| B�� | c����Һ�У�c��Na+����c��HCO3-����c��CO32-�� | |
| C�� | b����Һ�У�c��H+��=1��10-7mol•L-1 | |
| D�� | a����Һ�У�ˮ�������c��H+��=1��10-10mol•L-1 |
���� a��Ϊ��һ����������Һ��a��c������̼���ƣ�b����̼���ƺ��������ƵĻ����Һ��c���ǵ�һ��̼������Һ��c��d������̼�����ƣ�d����̼�����ƺ�̼��Ļ����Һ��d����Һ�����ԣ��ɴ˷������
A��d����Һ��ˮ�������OH-����Ũ��10-7mol/L����Һ�����ԣ���ϵ���غ�������
B����ˮ�������OH-����Ũ�����ʱ��˵����ʱ����Һ��̼������Һ����ˮ�ĵ�����ٽ����ã�
C�������£�c��OH-��=1��10-7mol/L����Һ�����ԣ�
D��ˮ������������������������ӵ�Ũ��ʼ����ȣ�a��û��ͨ������̼���������pH=10�� NaOH��Һ�����ˮ�����ӻ�����������⣮
��� �⣺A��d����Һ��ˮ�������OH-����Ũ��10-7mol/L����Һ�����ԣ�c��H+��=c��OH-������ϵ���غ�c��Na+��+c��H+��=2c��CO32-��+c��HCO3-��+c��OH-������ʽ�������c��Na+��=2c��CO32-��+c��HCO3-������A��ȷ��
B����ˮ�������OH-����Ũ�����ʱ��˵����ʱ����Һ��̼������Һ����ˮ�ĵ�����ٽ����ã�����������ҺŨ�ȴ�СΪc��Na+����c��CO32-����c��HCO3-������B����
C��ͼ���У�b���dz�����ˮ��Һ�е����������Ũ��c��H+��=c��OH-��=1��10-7mol/L����Һ�����ԣ���C��ȷ��
D��ˮ������������������������ӵ�Ũ��ʼ����ȣ�a��û��ͨ������̼���������pH=10�� NaOH��Һ����c��H+��=1��10-10mol/L������������Դ��ˮ�ĵ��룬����ˮ�������c��H+��=1��10-10mol/L����D��ȷ��
��ѡB��
���� ���⿼������ļ��㡢ͼ�����ݵĴ���֪�ȣ��Ѷ��еȣ�����ͼ���ж����ʼ����η����ķ�Ӧ�ǽ���Ĺؼ���

 ��ǰ����ϵ�д�
��ǰ����ϵ�д���NaOH��aq��+$\frac{1}{2}$H2SO4��Ũ���T$\frac{1}{2}$Na2SO4��aq��+H2O��l����H1
NaOH��aq��+CH3COOH��aq���TCH3COONa��aq��+H2O��l����H2
��CH4��g��+2O2��g���TCO2��g��+2H2O��g����H3
CH4��g��+2O2��g���TCO2��g��+2H2O��l����H4��
| A�� | ��H1����H2����H3����H4 | B�� | ��H1����H2����H3����H4 | C�� | ��H1=��H2����H3����H4 | D�� | ��H1����H2����H3����H4 |
| A�� | �⻯ѧ����������ŷ�̼�⻯����͵����������й� | |
| B�� | Ϊ��ֹ�����ն����������Ϲ�������֯Ҫ�������������CO2�Ĺ�ҵ�ŷ��� | |
| C�� | ������˿�ͽ��ڵ���Ҫ�ɷֶ�����ά�� | |
| D�� | Ϊ�˷�ֹ�����±��ȸ���֬ʳƷ�������ʣ��ӳ������ڣ�����װ���з�����ʯ�� |
| A�� | ��pH=12����Һ�У�Al3+��Cl-��HCO3-��Na+���Դ������� | |
| B�� | ��pH=0����Һ�У�Na+��NO3-��SO32-��K+���Դ������� | |
| C�� | ��0.1 mol/L-Ԫ��BOH��Һ��pH=10������֪BOH��Һ����BOH?B++OH- | |
| D�� | pH=2��һԪ���pH=12��һԪǿ�����������Һһ�����ڣ�c��OH-��=c��H+�� |
| A�� | ij���ʵ���Һ����ˮ�������c��H+��=1��10-amol/L����a��7ʱ�������Һ��pHһ��Ϊ14-a | |
| B�� | ��ͬ���ʵ���Ũ�ȵ�������Һ�У���NH4Al��SO4��2����NH4Cl����CH3COONH4����NH3•H2O��c��NH4+�� �ɴ�С��˳���ǣ��٣��ڣ��ۣ��� | |
| C�� | ���ʵ���Ũ����ȵ�H2S��NaHS�����Һ�У�c��Na+��+c��H+��=c��S2-��+c��HS-��+c��OH-�� | |
| D�� | AgCl����Һ�д���ƽ�⣺AgCl��s��?Ag+��aq��+Cl-��aq���������м������� NaCl��ĩ��ƽ��������ƶ�����Һ�����ӵ���Ũ�Ȼ��С |
| A�� | NaHB�Ĵ���������ˮ�ĵ��� | |
| B�� | HB-ˮ�ⷽ��ʽ��HB-+H2O?H3O++B2- | |
| C�� | ��ͬ���ʵ���Ũ����Һ��pHֵ��NaHB��Na2B | |
| D�� | ��Һ������Ũ�ȴ�СΪ��c��Na+����c��HB-����c��OH-����c��H+�� |
| A�� | ��һ����ѧ��Ӧ�У���Ӧ��ת����Խ����ѧ��Ӧ����Խ�� | |
| B�� | ��Ӧ�ȵ��ڷ�Ӧ���������������������� | |
| C�� | pH��7����Һһ���Ǽ���Һ | |
| D�� | HCl�ζ���ˮ�ü��ȱ��÷�̪��ָʾ��Ч������ |
| A�� | 16g��O2��O3�Ļ�������к��еķ�����Ϊ0.5NA | |
| B�� | 2.3g��������װ�������ļ���ƿ����ȫȼ�գ�ת�Ƶĵ�����Ϊ0.1NA | |
| C�� | 100mL 12mol/LŨ����������MnO2���ȣ����ɵ�Cl2������Ϊ0.3NA | |
| D�� | ��״���£�22.4LSO3���еķ�����ΪNA�� |