��Ŀ����
11����֪A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E������A��B��C��ͬһ���ڵķǽ���Ԫ�أ�������DCΪ���ӻ����D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��AC2Ϊ�Ǽ��Է��ӣ��Dz�������ЧӦ����Ҫ���壮B��C���⻯��ķе������ͬ����������Ԫ���⻯��ķе�ߣ�����ȱEԪ�ػ�����Dz���D��Eλ��ͬ���壮���������������ش��������⣺������ʱ��A��B��C��D��E������Ӧ��Ԫ�ط��ű�ʾ����1��A��B��C�ǽ�������ǿ������˳��ΪN��O��C��
��2��B���⻯��ķ���ʽ��NH3��B���⻯������ˮ�ĵ��뷽��ʽΪNH3+H2O?NH3•H2O?NH4++OH-��
��3��д��������AC2�ĵ���ʽ��
 �����Ǽ��ԣ�����ԡ��Ǽ��ԡ������γɵķǼ��Է��ӣ�
�����Ǽ��ԣ�����ԡ��Ǽ��ԡ������γɵķǼ��Է��ӣ���4��B������������Ӧ��ˮ�����ϡ��Һ��D�ĵ��ʷ�Ӧʱ��B����ԭ����ͼۣ��÷�Ӧ�Ļ�ѧ����ʽ��
4Mg+10HNO3=4Mg��NO3��2+NH4NO3 +3H2O��
���� ������DCΪ���ӻ����D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��˵��D��C����һ���ڣ�DӦΪMgԪ�أ�AC2Ϊ�Ǽ��Է��ӣ��Dz�������ЧӦ����Ҫ���壬������ӦΪCO2����AΪCԪ�أ�CΪOԪ�أ���BΪNԪ�أ�����ȱEԪ�ػ�����Dz���D��Eλ��ͬ���壬��EΪCaԪ�أ���϶�ӦԪ�صĵ��ʺͻ�����������Լ���ĿҪ��ɽ����⣮
��� �⣺������DCΪ���ӻ����D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��˵��D��C����һ���ڣ�DӦΪMgԪ�أ�AC2Ϊ�Ǽ��Է��ӣ��Dz�������ЧӦ����Ҫ���壬������ӦΪCO2����AΪCԪ�أ�CΪOԪ�أ���BΪNԪ�أ�����ȱEԪ�ػ�����Dz���D��Eλ��ͬ���壬��EΪCaԪ�أ�
��1��ͬ������ԭ����������һ�����ܳ��������ƣ�NԪ��2p�ܼ�����3�����ӣ�Ϊ�����ȶ�״̬�������ϵͣ���һ�����ܸ���ͬ����������˼�ǣ��ʵ�һ��������С�����˳��ΪN��O��C��
�ʴ�Ϊ��N��O��C��
��2��BΪNԪ�أ���Ӧ���⻯��ΪNH3����������ˮ�����������һˮ�ϰ���һˮ�ϰ��ĵ��뷽��ʽΪ��NH3+H2O?NH3•H2O?NH4++OH-��
�ʴ�Ϊ��NH3��NH3+H2O?NH3•H2O?NH4++OH-��
��3��AC2ΪCO2��Ϊ���ۻ��������ʽΪ ��������̼Ϊֱ���ͽṹ�����ɼ��Լ��γɵķǼ��Է��ӣ�
��������̼Ϊֱ���ͽṹ�����ɼ��Լ��γɵķǼ��Է��ӣ�
�ʴ�Ϊ�� �����ԣ�
�����ԣ�
��4��N����ͼ�Ϊ-3��DΪMgԪ�أ���Ӧ����NH4NO3����Ӧ�ķ���ʽΪ��4Mg+10HNO3=4Mg��NO3��2+NH4NO3 +3H2O��
�ʴ�Ϊ��4Mg+10HNO3=4Mg��NO3��2+NH4NO3 +3H2O��
���� ���⿼��ṹ����λ�ù�ϵӦ�ã���Ŀ�Ѷ��еȣ��漰�����ܡ����ӽṹ���ӻ����������ʽ������������ԭ��Ӧ�ȣ��⻯��ķе������ͬ����������Ԫ���⻯��ķе�����ƶϵ�ͻ�ƿڣ�

��l�� C��s��+$\frac{1}{2}$O2��g��=CO��g����H=��H1
��2��2H2��g��+O2��g��=2H2O��g����H=��H2
�ɴ˿�֪ C��s��+H2O��g���TCO��g��+H2��g����H3�����H3���ڣ�������
| A�� | ��H1-��H2 | B�� | ��H1-$\frac{1}{2}$��H2 | C�� | 2��H1-��H2 | D�� | $\frac{1}{2}$��H2-��H1 |
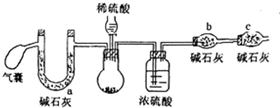 �������ƿ�������ɴ��Ĺ����������䴿��Ҫ��ܸߣ�ijС��ͬѧΪ�˲ⶨ�������ƵĴ��ȣ�����Ϊ̼���ƣ�����������·�����
�������ƿ�������ɴ��Ĺ����������䴿��Ҫ��ܸߣ�ijС��ͬѧΪ�˲ⶨ�������ƵĴ��ȣ�����Ϊ̼���ƣ�����������·���������һ��ȡm1g��Ʒ������ ������CaCl2��Һ����ַ�Ӧ����ˡ�ϴ�ӡ�������CaCO3��������Ϊm2g��
��������ȡm1g��Ʒ��������װ�ò�����ɶ�����̼����Ϊm3g��
��������ȡm1g��Ʒ����ˮ����ܽⲢ�������ٲ������壬��cmol/L���������Һ�ζ�������Һ��������ָʾ�������յ�ʱ������������ΪVmL��
�ش��������⣺
��1������һ�У�����ȷ������õĹ������ƵĴ��ȱ�ʵ�ʵ�ƫ�ͣ���ԭ���������ܵ�Ca��OH��2����ʹm2��ֵƫ��
��2��������������Ҫʹ�����Σ��ڶ���ʹ�õ�Ŀ�����ų�װ�������ɵ�CO2��ȫ����ʯ�����գ�C����ܵ������Ƿ�ֹ������ˮ�Ͷ�����̼����b�У�����ϡ�������ϡ���ᣬ��ⶨ���ƫ�ͣ���ߡ������͡���Ӱ�족��
��3���������У��ζ��յ����������Һ�ɻ�ɫ��ɳ�ɫ���Ұ�����ڲ���ƣ���ù������ƵĴ���Ϊ$\frac{39��0.053Vc-{m}_{1}��}{14{m}_{1}}$��100%�����ú�m1��c��V��ʽ�ӱ�ʾ��
��4��ijС��ͬѧ�����������ˮ��Ӧ����Һ�еμӷ�̪��������Һ�ȱ�����ɫ����Ե�����Һ��ɫ��ԭ��������ּ��裺
����һ��������������Һ��Ũ�ȹ����ʹ��Һ��ɫ
��������������˹��������ʹ��Һ��ɫ
��
ʵ����֤��������Ũ�ȷֱ�Ϊ5mol•L-1��2mol•L-1��1mol•L-1��0.01mol•L-1������������Һ�еμӷ�̪��Һ���۲쵽��Һ������ɫ��ʱ�����£�
| ��������Ũ�ȣ�mol•L-1�� | 5 | 2 | 1 | 0.01 |
| ������ɫ��ʱ�䣨s�� | 8 | 94 | 450 | ��ʱ�䲻��ɫ |
���ʵ����֤�����ȡ���ݵ����ķ�ӦҺ���Թ��У�������һ֧�Թܼ��������������̲��ȣ��μ��η�̪����Һ����Ҳ���ɫ����һ֧�Թ���ֱ�Ӽ��뼸�η�̪����Һ��������ɫ��˵�������������
| A�� | C3H8 | B�� | C4H10 | C�� | C5H12 | D�� | C6H14 |
| A�� | C2H6��C2H4O | B�� | C2H4O��C2H4O2 | C�� | C2H6O��C3H6O3 | D�� | C3H8O3��C2H4O2 |
| A�� | Ԫ�� | B�� | ԭ�� | C�� | ���� | D�� | ���� |
| A�� | ����������������֮�仯Ϊͭ�����ù��̷������û���Ӧ | |
| B�� | �����������Ѽ�·�ˡ����������γɵ����ܽ��ж����ЧӦ | |
| C�� | ������һ�գ���ˮ�����գ���ȡ֭���������϶������ص���ȡ���ڻ�ѧ�仯 | |
| D�� | �Ž�����¬�����Լ���Ϊ�У�����Ϊ���ɣ����������ۡ�������ָ�������ĺϽ� |
 ��
��
 ��
�� ��
��