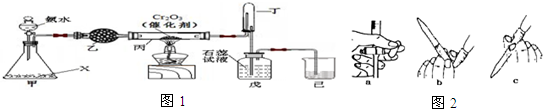
��Ŀ����
4�������ĸ�װ��ͼ����绯ѧ�йأ������ͼʾ�ش�������⣺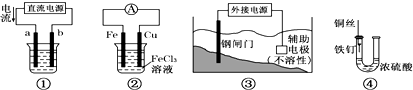
��1�����ĸ�װ���У����õ��ԭ�����Ǣ٢ۣ���װ����ţ���
��2��װ�â�����������ͭ����a���ĵ缫�����Ǵ�ͭ�����ͭ����ͭ�������������ҺΪCuSO4��Һ��
��3��װ�âڵ��ܷ�Ӧ����ʽ��Fe+2FeCl3�T3FeCl2��
��4��װ�â��и�բ��Ӧ����ӵ�Դ�ĸ��������������������
��5��װ�â��е���������û����ʴ����ԭ��������Ũ�������ˮ���ã�ʹU���еĿ����ܸ������������û����ʴ��
���� ��1������ӵ�Դ��װ�����ڵ��أ�
��2����⾫��ͭʱ����ͭ������������ͭ��ҺΪ�������Һ��
��3��Fe���Ȼ�����Һ��Ӧ�����Ȼ�������
��4������ӵ�Դ�ĸ�������ʱ��������������
��5��Ũ���������ˮ�ԣ��ڸ���Ļ����������ܷ����绯ѧ��ʴ��
��� �⣺��1��װ�â٢۶�����ӵ�Դ�����ڵ��أ������˵��ԭ����
�ʴ�Ϊ���٢ۣ�
��2��װ�â�����������ͭ�������ӵ�Դ����������������a�缫����aΪ��������⾫��ͭʱ����ͭ������������a���ĵ缫�����Ǵ�ͭ������ͭ���ӵ���ҺΪ�������Һ�������������ͭ��Һ���������Һ��
�ʴ�Ϊ����ͭ��CuSO4��Һ��
��3��װ�â��У�FeΪ����ʧ���������������ӣ�CuΪ�����������������ӵõ��������������ӣ����ܷ�Ӧ��ΪFe���Ȼ�����Һ��Ӧ�����Ȼ��������ܷ�Ӧ����ʽ��Fe+2FeCl3�T3FeCl2��
�ʴ�Ϊ��Fe+2FeCl3�T3FeCl2��
��4������ӵ�Դ�ĸ�������ʱ����������������������ʴ������װ�â��и�բ��Ӧ����ӵ�Դ�ĸ���������
�ʴ�Ϊ������
��5��Ũ���������ˮ�ԣ�Ũ���������տ����е�ˮ������ʹ������Χ�Ŀ�����ø���ڸ���Ļ����������ܷ����绯ѧ��ʴ��������������û����ʴ��
�ʴ�Ϊ������Ũ�������ˮ���ã�ʹU���еĿ����ܸ������������û����ʴ��
���� ���⿼����ԭ���ԭ���͵���ԭ���������ĵ绯ѧ��ʴ�ͷ����������ڿ���ѧ���Ի���֪ʶ��Ӧ����������Ŀ�ѶȲ���ע����յ缫���жϺ͵缫����ʽ����д��

| A�� | NO����������NO2��ͨ��װ��ˮ��ϴ��ƿ | |
| B�� | SO2�л�������HCl���壺ͨ������NaHSO3��Һϴ�� | |
| C�� | O2����������CO2��ͨ��װ�м�ʯ�ҵ�U�ι� | |
| D�� | ʳ������������NaHCO3���ӹ������ռ���Һ��������� |
| A�� | MgƬ���������缫��Ӧ��Mg-2e-�TMg2+ | |
| B�� | AlƬ���������缫��Ӧ��Al+4OH--3e-�TAlO2-+2H2O | |
| C�� | ���Ӵ�Mg�缫�ص�������Al�缫 | |
| D�� | AlƬ�������ݲ��� |

| A�� | �ɼ�֪��ʹ�ô�����Ӱ�췴Ӧ�� | |
| B�� | ���ҿ�֪�����ں��º��������µķ�Ӧ2NO2��g��?N2O4��g����A��Ϊƽ��״̬ | |
| C�� | �ɱ���֪��ͬ�¶ȡ�ͬŨ�ȵ�NaA��Һ��NaB��Һ��ȣ���pHǰ��С�ں��� | |
| D�� | �ɶ���֪����T1���A��B������Һ������T2��ʱ��A��B��Һ������������� |
| A�� | 100mL 0.5 mol•L-1MgCl2��Һ | B�� | 100mL 0.5mol•L-1KClO3��Һ | ||
| C�� | 200mL 0.25 mo l•L-1 KCl��Һ | D�� | 100mL 0.5mol•L-1 HCl��Һ |
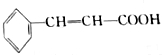 Ҳ���ڷ�ʽ�ṹ��1mol
Ҳ���ڷ�ʽ�ṹ��1mol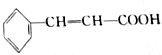 ��������4molH2�����ӳɷ�Ӧ��
��������4molH2�����ӳɷ�Ӧ��