��Ŀ����
12��Ŀǰ�뵼������չ����һ����ͭоƬ������-�ڹ�оƬ����ͭ���������ߣ����ϵĽ���ͭ���ִ��Ƽ�Ӧ����ȡ����ͻ�ƣ��û�ͭ����Ҫ�ɷ�ΪCuFeS2��������ͭ���䷴Ӧԭ�����£�
��1����̬��ԭ�ӵ���Χ�����Ų�ʽΪ3d104s1����Ԫ������Ԫ����ȣ���һ�����ܽϴ��Ԫ����O����Ԫ�ط��ţ���
��2����Ӧ�١����о���������ͬ��������ӣ��÷��ӵ�����ԭ���ӻ�������sp2��������ṹ��V�ͣ�
��3��ijѧ��������ͭ��Һ�백ˮ����һ��ʵ�飺CuSO4��Һ$\stackrel{��ˮ}{��}$��ɫ����$\stackrel{��ˮ}{��}$�����ܽ⣬�õ�����ɫ����Һ��д����ɫ�������ڰ�ˮ�����ӷ���ʽCu��OH��2+4NH3•H2O=[Cu��NH3��4]2++2OH-+4H2O������ɫ����Һ�е������ӣ�������H+���ڴ��ڵ�ȫ����ѧ�������й��ۼ�����λ����
���� ��1��Cuλ�ڵ������ڢ���B�壬��29��Ԫ�أ���̬ͭԭ�ӵļ۵����Ų�ʽΪ1s22s22p63s23p63d104s1��ͬ����Ԫ�ص�һ���������϶�����С��
��2���ɣ�1������֪��Ӧ�٢����ɵ���ͬ���������SO2��SO2�м۲���ӶԸ���=2+$\frac{1}{2}$��6-2��2��=3���Һ���һ���µ��Ӷԣ�������ռ乹����V�ͣ�Sԭ�Ӳ���sp2�ӻ���
��3������ͭ��Һ�백ˮ����������ͭ��������ͭ���ڹ����İ�ˮ���γ�[Cu��NH3��4]2+���ӣ������д��ڹ��ۼ�����λ����
��� �⣺��1��Cuλ�ڵ������ڢ���B�壬��29��Ԫ�أ���̬ͭԭ�ӵļ۵����Ų�ʽΪ3d104s1��ͬ����Ԫ�ص�һ���������϶�����С�����Ե�һ�����ܽϴ��������
�ʴ�Ϊ��3d104s1��O��
��2���ɣ�1������֪��Ӧ�٢����ɵ���ͬ���������SO2��SO2�м۲���ӶԸ���=2+$\frac{1}{2}$��6-2��2��=3������Sԭ�Ӳ���sp2�ӻ������ں���һ���µ��Ӷԣ���ռ乹����V�ͣ�
�ʴ�Ϊ��sp2��V�ͣ�
��3������ͭ��Һ�백ˮ����������ͭ��ɫ������������ͭ���ڹ����İ�ˮ���γ�[Cu��NH3��4]2+���ӣ���ɫ�������ڰ�ˮ�����ӷ���ʽΪCu��OH��2+4NH3•H2O=[Cu��NH3��4]2++2OH-+4H2O������ɫ����Һ�е������ӣ�������H+���ڴ��ڵ�ȫ����ѧ�������й��ۼ�����λ����
�ʴ�Ϊ��Cu��OH��2+4NH3•H2O=[Cu��NH3��4]2++2OH-+4H2O�����ۼ�����λ����
���� ���⿼������λ������ɼ��������Ŀ�Ѷ��еȣ��漰��������Ų����縺�ԡ���λ�������֪ʶ����ȷ��λ������ijɼ����Ϊ���ؼ�������������ѧ�������Ӧ��������

 ��ĩ��ϰ���ϵ�д�
��ĩ��ϰ���ϵ�д� ����ѧ�䵥Ԫ������ĩר����100��ϵ�д�
����ѧ�䵥Ԫ������ĩר����100��ϵ�д� �Ƹ�360�ȶ����ܾ�ϵ�д�
�Ƹ�360�ȶ����ܾ�ϵ�д� ���⿼����Ԫ���Ծ�ϵ�д�
���⿼����Ԫ���Ծ�ϵ�д�| A�� | ����ȼ�յ��Ȼ�ѧ����ʽ�ɱ�ʾΪ��C3H8��g��+5O2��g��=3CO2��g��+4H2O��g������H=-2221.5 kJ•mol-1 | |
| B�� | ������ȼ�յ��Ȼ�ѧ����ʽ�ɱ�ʾΪ��2C4H10��g��+18O2��g��=8CO2��g��+10H2O��l������H=-2878 kJ•mol-1 | |
| C�� | ������ת��Ϊ�춡��Ĺ�����һ�����ȹ��� | |
| D�� | ��������춡���ȶ� |

| A�� | ��͢� | B�� | ��͢� | C�� | ��͢� | D�� | ��͢� |
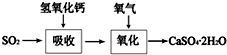 ��ҵ�ϳ����������ķ�������ˮ�����������ȣ�����������ã������ŷţ��������Ⱦ���������ѧ���գ��ɱ��Ϊ����
��ҵ�ϳ����������ķ�������ˮ�����������ȣ�����������ã������ŷţ��������Ⱦ���������ѧ���գ��ɱ��Ϊ����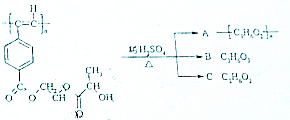 ����Ȳ����ۺ���ĺϳ�ʹ�߷��Ӳ��Ͻ����ˡ��ϳɽ����������ϵ���ѧʱ������ͼ�Ǿ���Ȳ���������M�Ľṹʽ��M��ϡ���������µ�ˮ��ʾ��ͼ��
����Ȳ����ۺ���ĺϳ�ʹ�߷��Ӳ��Ͻ����ˡ��ϳɽ����������ϵ���ѧʱ������ͼ�Ǿ���Ȳ���������M�Ľṹʽ��M��ϡ���������µ�ˮ��ʾ��ͼ��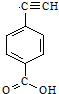 ��
��