��Ŀ����
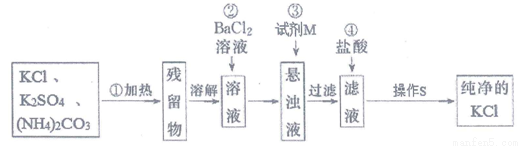
20����������Ƥ�﹤ҵ�й㷺Ӧ�ã�ijѧϰС�������ң���Ҫ�ɷ���Al��A12O3��FeO��SiO2�ȣ�Ϊԭ���Ʊ�������[Al��OH��2Cl]����ƹ���������ͼ��ʾ��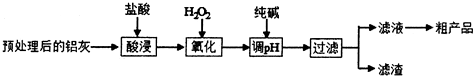
��֪��
| �������� | ��ʼ������pH | ��ȫ������pH |
| Fe3+ | 1.1 | 2.8 |
| A13+ | 3.4 | 4.7 |
| Fe2+ | 5.8 | 8.8 |
��1�����ʱ�¶ȿ�����30��-35�棬����̫�ͣ�Ҳ����̫�ߣ���ԭ�����¶�̫�ͣ���Ӧ�����¶�̫�ߣ�����ӷ��죮
��2������ʱ�����ӷ���ʽΪ2Fe2++H2O2+2H+=2Fe3++2H2O��
��3��������Һ��pH��ΧΪ2.8��pH��3.4��
��4����������Ҫ�������ʵĻ�ѧʽ��SiO2��Fe��OH��3��
���� ��1������������лӷ��Ժ��¶ȶԷ�Ӧ���ʵ�Ӱ��Ƕȷ�����
��2��ʵ��Ŀ�����Ʊ�������[Al��OH��2Cl]������Ҫ��ȥ�����Ӻ��������ӣ���������������������pH�ϸߣ���Ҫ��˫��ˮ������������ȫת���������ӣ��ݴ�д����Ӧ�����ӷ���ʽ��
��3�����ݱ�����������ȫ�����������ӿ�ʼ������pH���ݷ���������Һ��pH��Χ��
��4��������ҺpH����������ȫת�������������������ٽ�϶������費�����ᷴӦ��֪�����ijɷ֣�
��� �⣺��1�����¶�̫�ͣ���Ӧ�����¶�̫�ߣ�����ӷ��죬�����ܡ��¶ȿ�����30�桫35�棬����̫�ͣ�Ҳ����̫�ߣ�
�ʴ�Ϊ���¶�̫�ͣ���Ӧ�����¶�̫�ߣ�����ӷ��죻
��2���Ʊ�������[Al��OH��2Cl]������Ҫ��ȥ�����Ӻ��������ӣ���������������������pH�ϸߣ����������ӣ�����Ҫ��˫��ˮ������������ȫת���������ӣ���Ӧ�����ӷ���ʽΪ��2Fe2++H2O2+2H+=2Fe3++2H2O��
�ʴ�Ϊ��2Fe2++H2O2+2H+=2Fe3++2H2O��
��3�����ݱ������ݿ�֪��pH=2.8ʱFe3+��ȫ������pH=3.4ʱA13+��ʼ������Ҫ��ȥ��Һ�е������Ӷ�����ʹ�����ӳ�����Ҫ����Һ��pH��ΧΪ��2.8��pH��3.4��
�ʴ�Ϊ��2.8��pH��3.4��
��4���ô��������ҺpH����������ȫת����Fe��OH��3�����������к���Fe��OH��3������SiO2�������ᷴӦ������������Ҫ�ɷ�ΪSiO2��Fe��OH��3��
�ʴ�Ϊ��SiO2��Fe��OH��3��
���� ���⿼�����Ʊ���������ƣ���Ŀ�Ѷ��еȣ������Ʊ���������ʵ��Ŀ�ġ�ʵ��ԭ��Ϊ���ؼ�����2��Ϊ�״��㣬ע���������������ʼ����������������ȫ������pH����������������ѧ���ķ�����������ѧʵ��������

 ij��ѧʵ��С����Ҫ�˽��г�������ʳ�ð״ף���Ҫ�Ǵ����ˮ��Һ������ȣ���ȵ�λ��g/100ml�����ִ��г�������һƿijƷ��ʳ�ð״ף���ʵ���ұ�NaOH��Һ������еζ����±���4�ֳ���ָʾ���ı�ɫ��Χ��
ij��ѧʵ��С����Ҫ�˽��г�������ʳ�ð״ף���Ҫ�Ǵ����ˮ��Һ������ȣ���ȵ�λ��g/100ml�����ִ��г�������һƿijƷ��ʳ�ð״ף���ʵ���ұ�NaOH��Һ������еζ����±���4�ֳ���ָʾ���ı�ɫ��Χ��| ָʾ�� | ʯ�� | ���� | ���� | ��̪ |
| ��ɫ��Χ ��PH�� | 5.0-8.0 | 3.1-4.4 | 4.4-6.2 | 8.2-10.0 |
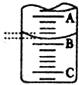
��2����ͼ��ʾ50ml�ζ�����Һ���λ�ã���A��C�̶ȼ����1ml��A���Ŀ̶�Ϊ25���ζ�����Һ�����Ϊ25.40ml����ʱ�ζ�����Һ����������24.60mL��
��3��Ϊ�˼�Сʵ������ͬѧһ������������ʵ�飬����ÿ����ȡ�״������ΪVml��NaOH��ҺŨ��ΪC mol/L������ʵ������¼���£�
| ʵ����� | ��һ�� | �ڶ��� | ������ |
| ����NaOH��Һ���/ml | 26.02 | 25.32 | 25.28 |
A��ʵ�����ʱ���ӿ̶��߶�ȡ�ζ��յ�ʱNaOH��Һ�����
B���ζ�ǰ�ζ��ܼ��������ݣ��ζ�����������
C��ʢװ��Һ�ĵζ���װҺǰ������ˮ��ϴ����δ�ñ�Һ��ϴ
D����һ�εζ��õ���ƿ�ô�װҺ��ϴ����������δ��ϴ
E���ζ�NaOH��Һ���죬δ������տ�����Һ��ɫ������ֹͣ�ζ�
��4�������������ݣ�д������ð״�������ȵı���ʽ�����Բ��ػ���$\frac{��25.28+25.32����c��0.1��60}{2V}$
����ȣ�ÿ100ml��Һ�к������������������λ��g/100ml��
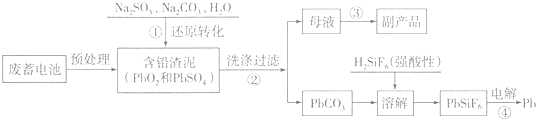
��֪��Ksp��PbSO4��=1.6��10-5��Ksp��PbCO3��=3.3��10-14��
�ش��������⣺
��1��д���������PbSO4ת��ΪPbCO3���̵�ƽ�ⳣ������ʽK=$\frac{c��S{{O}_{4}}^{2-}��}{c��C{{O}_{3}}^{2-}��}$��Ϊ��߲���ٵķ�Ӧ���ʺ�Ǧ�����ʣ�����Ϊ�ɲ�ȡ��������ʩ�dz�ֽ��衢�ʵ������¶ȣ�
��2��������з�����������ԭ��Ӧ�����ӷ���ʽΪPbO2+SO32-+H2O=PbSO4+2OH-��
��3��д��������ö��Ե缫����������ӦʽPb2++2e-=Pb��
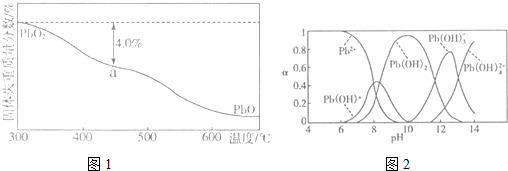
��4��PbO2�ڼ��ȹ��̷����ֽ��ʧ��������ͼ1��ʾ����֪ʧ�������ϵ�a��Ϊ��Ʒʧ��4.0%����$\frac{��Ʒ��ʼ����-a���������}{��Ʒ��ʼ����}$��100%���IJ������壬��a�������ɱ�ʾΪPbOx������x=1.4��
��5��Ǧ�ļӹ�ͬ����ʹˮ�����ؽ���Ǧ�ĺ����������������Ⱦ��ˮ��Һ��Ǧ�Ĵ�����̬��Ҫ��Pb2+��Pb��OH��+��Pb��OH��2��Pb��OH��3-��Pb��OH��42-������̬��Ũ�ȷ���������ҺpH�仯�Ĺ�ϵ��ͼ2��ʾ��ij�������Ʊ���һ��������Ǧ��������Чȥ��ˮ�еĺ���Ǧ��ʵ�������
| ����/��mol��L-1�� | Pb2+ | Ca2+ | Fe3+ | Mn2+ | Cl- |
| ����ǰŨ�� | 0.100 | 29.8 | 0.120 | 0.087 | 51.9 |
| ������Ũ�� | 0.004 | 22.6 | 0.040 | 0.053 | 49.9 |
A��4��5 B��6��7 C��9��10 D��11��12��
| A�� | 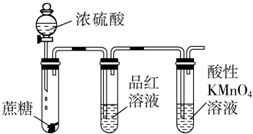 ����Ũ���������Ƿ�Ӧ�����Ķ������� | |
| B�� | 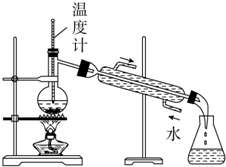 ����ױ���ˮ | |
| C�� | 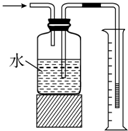 ������������� | |
| D�� | 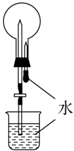 ���ж�����̼��Ȫʵ�� |
 2NH3��+CO2��+H2O
2NH3��+CO2��+H2O