��Ŀ����
7������A������һϵ�з�Ӧ�����ղ���Ϊ���ᣨHOOC-COOH����
��֪��B����Է���������A��34.5����ش��������⣺
��1��д���������ʵĽṹ��ʽ��B��CH3CH2Cl E��CH2OH-CH2OH F��OHC-CHO��
��2��д��ָ�����йط�Ӧ�ķ����ͣ�A��B��ȡ����Ӧ��B��C����ȥ��Ӧ��E��F��������Ӧ��
��3��B��C�ķ�Ӧ����Ϊ���������ƴ���Һ������
��4��д��D��E��Ӧ�Ļ�ѧ��Ӧ����ʽ��CH2BrCH2Br+2NaOH $��_{��}^{H_{2}O}$CH2OHCH2OH+2NaBr��
��5��E��F�Ļ�ѧ��Ӧ����ʽ��CH2OH-CH2OH+O2$��_{��}^{Cu}$OHC-CHO+2H2O��
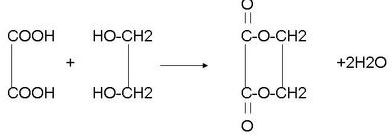
��6��E����ᷴӦ���ɻ�״��Ļ�ѧ��Ӧ����ʽ��
 ��
��
���� A��CH3CH3��B����Է���������A��34.5����A����������ȡ����Ӧ����B����B��CH3CH2Cl��B���������ƵĴ���Һ������ȥ��Ӧ������ϩ����C��CH2=CH2��F����������HOOC-COOH����F��OHC-CHO��E��������OHC-CHO����E��CH2OHCH2OH��C���巢���ӳɷ�Ӧ����D��D���������Ƶ�ˮ��Һ����ȡ����Ӧ����E����D��CH2BrCH2Br���ݴ˷������
��� �⣺A��CH3CH3��B����Է���������A��34.5����A����������ȡ����Ӧ����B����B��CH3CH2Cl��B���������ƵĴ���Һ������ȥ��Ӧ������ϩ����C��CH2=CH2��F����������HOOC-COOH����F��OHC-CHO��E��������OHC-CHO����E��CH2OHCH2OH��C���巢���ӳɷ�Ӧ����D��D���������Ƶ�ˮ��Һ����ȡ����Ӧ����E����D��CH2BrCH2Br��
��1���������Ϸ�����B��CH3CH2Cl��E��CH2OHCH2OH��F��OHC-CHO��
�ʴ�Ϊ��CH3CH2Cl��CH2OH-CH2OH��OHC-CHO��
��2���������Ϸ�����A��B��ȡ����Ӧ��B��C����ȥ��Ӧ��E��F��������Ӧ��
�ʴ�Ϊ��ȡ����Ӧ����ȥ��Ӧ��������Ӧ��
��3��B��C��B���������ƵĴ���Һ������ȥ��Ӧ������ϩ����Ӧ����Ϊ���������ƴ���Һ�����ȣ�
�ʴ�Ϊ���������ƴ���Һ�����ȣ�
��4��D��CH2BrCH2Br��CH2BrCH2Br���������Ƶ�ˮ��Һ�ڼ��������·���ȡ����Ӧ����CH2OHCH2OH����Ӧ����ʽΪ��CH2BrCH2Br+2NaOH $��_{��}^{H_{2}O}$CH2OHCH2OH+2NaBr��
�ʴ�Ϊ��CH2BrCH2Br+2NaOH $��_{��}^{H_{2}O}$CH2OHCH2OH+2NaBr��
��5��CH2OH-CH2OH��������OHC-CHO����Ӧ�Ļ�ѧ����ʽΪ��CH2OH-CH2OH+O2 $��_{��}^{Cu}$ OHC-CHO+2H2O��
�ʴ�Ϊ��CH2OH-CH2OH+O2 $��_{��}^{Cu}$ OHC-CHO+2H2O��
��6��EΪCH2OHCH2OH����HOOC-COOH������Ӧ���ɻ�״���Ӧ�ķ���ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
���� ���⿼�����л�����ƶϣ���Ŀ�Ѷ��еȣ���HOOC-COOHΪͻ�ƿڲ������Ƶķ��������ƶϣ���ȷ�л���Ľṹ�������ǽⱾ��ؼ���ע�ⷴӦ��������Ӧ������ͬ���²��ﲻͬ��Ϊ�״��㣬����������ѧ���ķ���������������������

 ��ĩ�óɼ�ϵ�д�
��ĩ�óɼ�ϵ�д� 99��1������ĩ��ѵ��ϵ�д�
99��1������ĩ��ѵ��ϵ�д� ��ǿ��У��ĩ���100��ϵ�д�
��ǿ��У��ĩ���100��ϵ�д�| A�� | С�մ����������۷��� | B�� | SO2������Ư��ֽ�� | ||
| C�� | BaCO3��ҽѧ���������� | D�� | �����ڳ�ȥůˮƿ�е�ˮ�� |
�������ϣ�Cu2O��һ�ּ������������ϡ��������Cu��CuSO4��Cu2O�ڿ����м�������CuO
�������
����1����ɫ��ĩ��Fe2O3
����2����ɫ��ĩ��Cu2O
����3����ɫ��ĩ��Fe2O3��Cu2O�Ļ����
���̽��ʵ��
ȡ������ĩ��������ϡ�����У���������Һ���ٵμ� KSCN �Լ���
��1��������1��������ʵ����������Һ��ΪѪ��ɫ��
��2�����μ� KSCN �Լ�����Һ�����ɫ����֤��ԭ�����ĩ��һ����������������������Ϊ����˵�������𣿲�������
��3���������ĩ��ȫ�ܽ�������ڣ��μ� KSCN �Լ�ʱ��Һ�����ɫ����֤��ԭ�����ĩ��Fe2O3��Cu2O�Ļ���д��������Ӧ�����ӷ���ʽFe2O3+6H+=2Fe3++3H2O��Cu2O+2H+=Cu+Cu2++H2O��2Fe3++Cu=2Fe2++Cu2+��
̽������
��ʵ�������ȷ����ɫ��ĩΪFe2O3��Cu2O�Ļ���
��4��ʵ��С�����ü��ȷ��ⶨCu2O������������ȡa g�����ĩ�ڿ����г�ּ��ȣ����������ٱ仯ʱ����������Ϊbg��b��a������������Cu2O����������Ϊ$\frac{9��b-a��}{a}��100%$��
��5�������ú�ɫ��ĩFe2O3��Cu2O�Ļ������ȡ�ϴ����ĵ�����CuSO4?5H2O�������������ϵ�֪������Һ�е�����Һ������Զ�ʹCu2+��Fe2+��Fe3+�ֱ����ɳ�����pH
| ���� | Cu��OH��2 | Fe��OH��2 | Fe��OH��3 |
| ��ʼ����pH | 6.0 | 7.5 | 1.4 |
| ������ȫpH[���� | 13 | 14 | 3.7 |
A����ˮ
B��H2O2
C������
D��NaOH
E����ˮ
F��Cu2��OH��2CO3
ʵ��С���������ʵ�鷽����

�Իش𣺢��Լ�IΪB���Լ�IIΪF������ĸ����
�ڹ���X�Ļ�ѧʽΪFe��OH��3��
| A�� | CH3COOH+OH-�TCH3COO-+H2O | B�� | H++OH-�TH2O | ||
| C�� | CH3COOH+OH-+Na+�TCH3COONa+H2O | D�� | CH3COOH+NaOH�TCH3COO-+Na++H2O |
| A�� | �������;ƾ� | B�� | �屽���� | C�� | �ױ���ˮ | D�� | �������ͼ��� |


 ��
�� ��
��
 ��
��
 ����
���� ��
�� ����
����