��Ŀ����
4�����1����Ӧ���Էּ������У�����ֲ���Ӧ�ķ�Ӧ��֮����÷�Ӧһ�����ʱ�ķ�Ӧ������ͬ�ģ�������ɳ�Ϊ��˹���ɣ��ݴ˻ش��������⣺��1���������˻ᡰ���ơ����ȼ���DZ��飨C3H8��������������˻���ȼ���DZ�ϩ��C3H6������������ɵñ�ϩ��
��֪����CH3CH2CH3��g����CH4��g��+HC��CH��g��+H2��g������H1=+156.6kJ•mol-1?
��CH3CH=CH2��g����CH4��g��+HC��CH��g������H2=+32.4kJ•mol-1?
����ͬ�����£���������ñ�ϩ���Ȼ�ѧ����ʽΪ��C3H8��g����CH3CH=CH2��g��+H2��g����H=+124.2KJ/mol��
��2�������飨B2H6����һ����̬����ȼ�ϣ�0.3mol����������������ȼ�գ����ɹ�̬�����������Һ̬ˮ���ų�649.5kJ���������Ȼ�ѧ��Ӧ����ʽΪB2H6��g��+3O2��g��=B2O3��s��+3H2O��l����H=-2165kJ/mol��
��H2O��l��=H2O��g������H=+44kJ/mol����5.6L���������������ȫȼ��������̬ˮʱ���ų���������508.25 kJ��
���� ��1�����������Ȼ�ѧ����ʽ��˹����д����������ñ�ϩ���Ȼ�ѧ����ʽ��
��2�������Ȼ�ѧ����ʽ����д������֪�����ʵ����ʵ����뷴Ӧ�ų������������ȣ���ע����������ʵľۼ�״̬�����
�ȸ��ݸ�˹����д������ʽ��Ȼ��������ʵ����ʵ����뷴Ӧ�ų������������������
��� �⣺��1����C3H8��g����CH4��g��+HC��CH��g��+H2��g����H1=+156.6kJ•mol-1
��CH3CH=CH2��g����CH4��g��+HC��CH��g����H2=+32.4kJ•mol-1
���ݸ�˹���ɢ�-�ڿɵã�C3H8��g����CH3CH=CH2��g��+H2��g����H=+124.2KJ/mol��
�ʴ�Ϊ��C3H8��g����CH3CH=CH2��g��+H2��g����H=+124.2KJ/mol��
��2��0.3mol��̬����ȼ����������������ȼ�գ����ɹ�̬�����������Һ̬ˮ���ų�649.5KJ����������1mol��̬����ȼ����������������ȼ�գ����ɹ�̬�����������Һ̬ˮ���ų�2165KJ����������Ӧ���Ȼ�ѧ����ʽΪ��B2H6��g��+3O2��g��=B2O3��s��+3H2O��l����H=-2165kJ/mol��
���ݣ�B2H6��g��+3O2��g��=B2O3��s��+3H2O��l����H=-2165kJ/mol�� ��
H2O��l����H2O��g������H=+44kJ/moL����
��+�ڡ�3�ã�B2H6��g��+3O2��g��=B2O3��s��+3H2O��g����H=-2033kJ/mol����5.6L����״������0.25mol��������ȫȼ��������̬ˮʱ�ų���������Ϊ2033kJ��0.25=508.25kJ��
�ʴ�Ϊ��B2H6��g��+3O2��g��=B2O3��s��+3H2O��l����H=-2165kJ/mol��508.25��
���� ������Ҫ�������Ȼ�ѧ����ʽ����д����˹���ɵ������Լ������ļ��㣬��Ҫע����Ƿ�Ӧ�ȵ���ֵ�뻯ѧ����ʽǰ���ϵ�������ȣ���Ŀ�Ѷ��еȣ������ڿ���ѧ���ķ��������ͼ���������

 ��ѧȫ��������ѵ��ϵ�д�
��ѧȫ��������ѵ��ϵ�д�| A�� | Al2��SO4��3 | B�� | Na2CO3 | C�� | AlCl3 | D�� | KNO3 |
| A�� | �Ͻ� | B�� | �����β��� | C�� | �л��߷��Ӳ��� | D�� | ���ǽ������� |
��2����֪H2��g��+Cl2��g���T2HCl��g����H=-184.6kJ•mol-1����������������±���
| H2��g�� | Cl2 ��g�� | HCl ��g�� | |
| 1mol�����еĻ�ѧ������ʱ��Ҫ���յ�����/kJ | 436 | 243 | a |
��3����֪��2SO2��g��+O2��g���T2SO3��g����H=-196.6kJ•mol-1
2NO��g��+O2��g���T2NO2��g����H=-113.0kJ•mol-1
��ӦNO2��g��+SO2��g���TSO3��g��+NO��g���ġ�H=-41.8kJ•mol-1��
| A�� | M��N | B�� | 2M=N | C�� | N��M | D�� | M=N |
| A�� | CO2��CO | B�� | Zn��Zn2+ | C�� | H2��H2O | D�� | CuO��CuCl2 |
| A�� | Clԭ�ӵĽṹʾ��ͼ��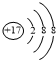 | B�� | ������Ľṹʽ��H-Cl-O | ||
| C�� | NH4Cl�ĵ���ʽ��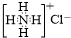 | D�� | �������ױ��Ľṹ��ʽ��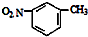 |
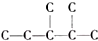 ����������һ�������6 �֣���������ΪȲ�������Ƶã����Ȳ���Ľṹ��ʽΪ��CH3��2CH��CH3��CHC��CH��
����������һ�������6 �֣���������ΪȲ�������Ƶã����Ȳ���Ľṹ��ʽΪ��CH3��2CH��CH3��CHC��CH��