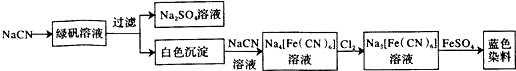
��Ŀ����
4��������������Ϊ98%���ܶ�Ϊ1.84g•cm-3��Ũ����������90mL��1mol•L-1��ϡ���ᣮ������������⣺��1����Ҫ�õ��IJ��������У��ձ�������������Ͳ��100mL����ƿ����ͷ�ι�
��2�������㣬��Ũ��������Ϊ5.4mL��
��3�������ƹ����У�����������ȷ�����в����д�����Ǣ٢ڢۢܢޢߣ����������ƫ�ߵ��Т٢ڢޢߣ�����ţ���
��ϴ����ȡŨ��������Ͳ������ϴ��Һת�Ƶ�����ƿ��
��δ��ϡ�ͺ��H2SO4��Һ��ȴ�����¾�ת�Ƶ�����ƿ��
�۽�Ũ����ֱ�ӵ����ձ��������ձ���ע������ˮ��ϡ��Ũ����
�ܶ���ʱ��������ˮ�������ߣ����ý�ͷ�ι�����
��ת��ǰ������ƿ�к�����������ˮ
����ȡŨ����ʱ�����ӱ���
�߶���ʱ�����ӱ��ߣ�
���� ��1������ʵ������IJ����Լ�ÿ��������Ҫ����ȷ����Ӧ����������
��2��ŨH2SO4�����ʵ���Ũ��c=$\frac{1000�Ѧ�}{M}$���ٸ�����Һϡ��ǰ�����ʵ��������������Ũ����������
��3����������һ�����ʵ���Ũ����Һ�IJ��������жϣ������������������ʵ����ʵ�������Һ�������Ӱ�죬����c=$\frac{n}{V}$������������
��� �⣺��1�����Ʋ�������ȡ��ϡ�͡���Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ���������Ͳ��ȡ���õ���ͷ�ιܣ����������ձ���ϡ�ͣ����ò��������裬��ȴ��ת�Ƶ�100mL����ƿ�У����ò���������������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ���Ҫʹ�õ������У��ձ�������������Ͳ��100mL����ƿ����ͷ�ιܵȣ�
��ѡ���ձ�������������Ͳ��100mL����ƿ����ͷ�ιܣ�
��2����������Ϊ98%���ܶ�Ϊ1.84g•cm-3��Ũ����C=$\frac{1000��1.84��98%}{98}$=18.4mol/L������ҪŨ�������ΪV���������Һϡ��ǰ�����ʵ�������ã�V��18.4mol/L=100mL��1mol/L�����V=5.4mL��
�ʴ�Ϊ��5.4mL��
��3����ϴ����ȡŨ��������Ͳ������ϴ��Һת�Ƶ�����ƿ�У�������������ʵ���ƫ����ҺŨ��ƫ�ߣ���������
��δ��ϡ�ͺ��H2SO4��Һ��ȴ�����¾�ת�Ƶ�����ƿ�У���ȴ����Һ���ƫС����ҺŨ��ƫ�ߣ���������
��Ũ����ϡ����ȷ��������Ũ���Ỻ��ע��ʢ��ˮ���ձ��У���������
�ܶ���ʱ��������ˮ�������ߣ����ý�ͷ�ι������������������ʵ���ƫС����ҺŨ��ƫ�ͣ���������
��ת��ǰ������ƿ�к�����������ˮ������Һ������������ʵ������������Ӱ�죬��ҺŨ�Ȳ��䣬������ȷ��
����ȡŨ����ʱ�����ӱ��ߣ�������ȡ����������ʵ���ƫ����ҺŨ��ƫ�ߣ���������
�߶���ʱ�����ӱ��ߣ�������Һ���ƫС����ҺŨ��ƫ�ߣ���������
�����д�����Ǣ٢ڢۢܢޢߣ����������ƫ�ߵ��Т٢ڢޢߣ�
�ʴ�Ϊ���٢ڢۢܢޢߣ��٢ڢޢߣ�
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƹ����еļ��������������ȷ����ԭ�������������ǽ���ؼ���ע������ƿ����ȷʹ�÷�����

| A�� | $\frac{12n}{m}$ | B�� | $\frac{60n}{m}$ | C�� | $\frac{11m}{120n}$ | D�� | $\frac{120n}{11m}$ |
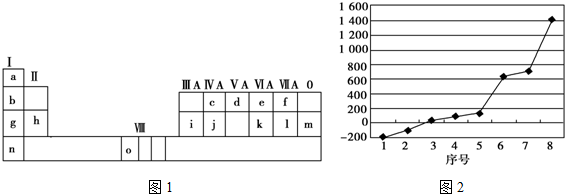
| Ԫ�ش��� | A | B | C | D | E |
| ԭ�Ӱ뾶/nm | 0.160 | 0.143 | 0.102 | 0.071 | 0.099 |
| ��Ҫ���ϼ� | +2 | +3 | +6��-2 | -1 | -1 |
| A�� | A��B�����Ӱ뾶��С��ϵΪ��B��A | |
| B�� | D��E�γɵļ����ӵĻ�ԭ�ԣ�E��D | |
| C�� | ��̬�⻯����ȶ��ԣ�D��C | |
| D�� | ����������Ӧ��ˮ��������ԣ�C��E |
| A�� | I2 | B�� | MgCl2 | C�� | KCl | D�� | H2O |
 Ŀǰ��ҵ���Ʊ���ϩ�棨CH2=CHC��N������Ȳ������ϩ���������ȣ�
Ŀǰ��ҵ���Ʊ���ϩ�棨CH2=CHC��N������Ȳ������ϩ���������ȣ�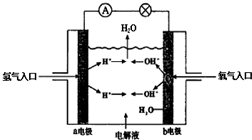
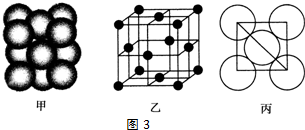
 ��
��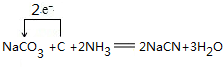 ��
��